Abstract
Until recently, radiotherapy was viewed solely from a tumour cell-autonomous perspective, whereby successful therapy resulted from inflicting breaks in nuclear DNA above an unspecified threshold that exceeded the tumour cell’s capacity for repair. Greater understanding of the importance of non-tumour cell-autonomous, immunological aspects of radiation-induced cell death in the context of the tumour micro-environment (TME) has altered this rather narrow perception. We now know that clinical responses to radiotherapy are inextricably linked to the immune system: loco-regional radiotherapy can trigger abscopal, immune-mediated responses at distant unirradiated sites (albeit rarely), while patients who are pathologically or iatrogenically immunosuppressed may derive less benefit from radiotherapy. The intrinsic biology of individual tumours, their associated microenvironments, and the physical characteristics of the delivered radiation, can all influence the immunogenicity of radiotherapy. By understanding and modulating cross-talk between molecular responses to radiation-induced DNA damage, associated mechanisms of cell death and subsequent innate and adaptive immune responses, we may be able to improve clinical outcomes of radiotherapy.
In this chapter, the focus will be on mechanisms of DNA damage repair and how tumours exploit alterations in these to enhance their survival. However, tumour cell-intrinsic aberrations in DNA repair can render tumour cells vulnerable to the effects of radiotherapy and this may be enhanced further by rational use of targeted DNA damage-response inhibitors. In particular, we will focus on how disordered DNA repair and its pharmacological modulation through ataxia telangiectasia and Rad3-related kinase inhibition can lead to radiation-induced immunostimulation and how this can be exploited further in the clinic through the use of specific immunotherapies, such as immune checkpoint blockers. As part of the discussion, specific mechanisms of radiation-induced cell death will be discussed, with emphasis on mechanisms of triggering immunologically visible, pro-inflammatory modes of cell death.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Ataxia telangiectasia and Rad3-related kinase
- Cell cycle checkpoint
- DNA damage repair
- Immune checkpoint inhibitor
- Immunotherapy
- Radiation
Introduction
Radiotherapy is one of the most effective and frequently used treatments for a variety of cancers. Approximately half of cancer patients receive radiotherapy at some point in their treatment [1], whether in the curative or palliative settings. Radiotherapy causes cell death or senescence via DNA damage. In general terms, necrotic or apoptotic cell death occurs depending on cell type, radiotherapy dose and fractionation schedule [2]. Cancer cells that evade apoptosis and continue to divide with accumulated DNA damage may die via mitotic catastrophe. Classically, the outcome of fractionated radiotherapy is governed by the principles of the 5 Rs of radiobiology, one of which is repair of DNA damage [3]. Therefore, combining radiation with agents that can target, and inhibit, DNA damage repair pathways represents an important new avenue towards enhanced therapeutic outcomes.
In addition to its direct anti-cancer cytotoxicity, ionising radiation can promote anti-tumour immune responses by triggering pro-inflammatory signals, DNA damage-induced immunogenic cell death (ICD) and innate immune activation. Anti-tumour innate immunity can arise from recruitment and stimulation of directly active natural killer (NK) cells. In addition, dendritic cells (DCs) can be recruited and activated with subsequent tumour-specific adaptive T-cell priming and immunostimulatory cell infiltration. The reverse effect can also occur through radiotherapy-induced immunosuppression and generation of anti-inflammatory mediators that can confer radioresistance. Approaches that target the DNA damage response (DDR) concomitantly with radiotherapy are attractive strategies for circumventing radioresistance, by enhancing the radiosensitivity of tumour relative to normal tissues, but also by re-programming the tumour microenvironment to create an immunostimulatory milieu. This doubly-targeted approach seeks to exploit tumour-intrinsic genomic instability as a means of preventing immune evasion.
Here, we review targeting of ataxia telangiectasia and Rad3-related kinase (ATR) and the potential this brings for interactions with druggable immunomodulatory signalling pathways, including nucleic acid-sensing mechanisms (Toll-like receptors (TLR); cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) and retinoic acid-inducible gene-I (RIG-I)-like receptors), and immune checkpoint inhibitors (ICPI). Central to these discussions are considerations of how these approaches might be exploited to enhance the effects of radiation therapy.
Immunostimulatory Effects Mediated by Radiotherapy
The innate immune system uses pattern-recognition receptors (PRRs) to detect microbial pathogenic molecules known as pathogen-associated molecular patterns (PAMPs). However, these pathways are not exclusively limited to foreign molecules and immune activation can also occur without microbial infection. In such cases, it may be triggered by inflammatory signals released from stressed or dying cells, collectively known as damage-associated molecular patterns (DAMPs) [4]. Radiotherapy-induced cellular stress and ICD can stimulate immune responses through the generation of DAMPs [5], which can be detected by their cognate PRRs [6]. ICD has been defined as the chronic expression of DAMPs in the tumour microenvironment (TME) and this can induce innate and adaptive anti-tumour immune responses in the host [7].
Classically, ICD-related DAMPs include: adenosine triphosphate (ATP) secretion; high-mobility group box-1 (HMGB1) protein release; and calreticulin expression on the cell surface. Extracellular ATP functions as a “find-me” chemoattractant signal [7] and promotes recruitment and activation of dendritic cells [8, 9]. HMGB1 protein, released from the nucleus during ICD, binds to TLR-4 and is critical for activating DCs and facilitating antigen-processing and presentation to T-cells [10]. Calreticulin exposure on the external surface of dying cells provides an “eat-me” signal to antigen-presenting cells (APCs) and results in their phagocytosing target cells [11]. ICD leads to release of tumour-associated antigens (TAA) and, subsequently, their acquisition, processing and presentation by APCs, potentially leading to priming of a cancer-specific adaptive immune response.
Radiotherapy-induced DNA damage can act as a viral mimic through the accumulation of cytosolic DNA or RNA in irradiated cells [12]. Cytosolic DNA and RNA activate cGAS-STING and RIG-I/mitochondrial antiviral-signalling protein (MAVS) pathways, respectively [13]. These pathways activate complex downstream signalling via interferon regulatory factor-3 (IRF-3)/TANK-binding kinase 1 (TBK1) and nuclear factor kappa B (NF-κB) that results in production of Type I interferon (IFN) and other inflammatory cytokines (e.g. interleukin [IL]-1, tumour necrosis factor [TNF]-α) [12]. Detailed consideration of all of these pathways is beyond the scope of this review, but there are a number of active programmes of research seeking to generate activators of cGAS-STING, RIG-I and TLR pathways to augment anti-tumour immune responses.
There are also data demonstrating that radiation can enhance cancer cell antigenicity through upregulation of genes involved in DNA damage repair and cellular stress responses [12]. Immune cell recruitment is increased via expression of adhesion molecules (e.g. intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and E-selectin) [14] and chemokines (e.g. CXCL16) [15]. Within the appropriate inflammatory environment, APCs take up antigens in peripheral tissues, mature and migrate to draining lymph nodes, where they activate naïve T-cells and promote their differentiation into effector T-cells [16]. Radiotherapy-induced ICD increases TAA presentation that can lead to specific T-cell priming, expansion of tumour reactive CD8+ T-cells and infiltration into the TME [17].
In summary, inflammatory DAMP signalling generates a favourable TME for activated DCs to process and cross-present TAAs from irradiated cells as a “tumour vaccine” to naïve T-cells. These primed and expanded T-cells can sustain a systemic tumour-specific immune response, in effect converting an initial innate to an adaptive anti-tumour response with the potential for durable, systemic activity and the development of long-lasting anti-tumour memory. The T-cell receptor (TCR) repertoire is also known to be shaped following radiotherapy, including when used in conjunction with ICPI [18,19,20].
Immunosuppressive Mechanisms Triggered by Radiotherapy
Pro-inflammatory signalling, as reviewed above, can trigger beneficial anti-tumour effects, but cancer cells learn to adapt and survive with mechanisms such as hypoxia resistance and unrestricted proliferation that can result in a state of chronic inflammation and evasion of immune surveillance [21,22,23]. Cancer cells can also adapt to down-regulate or lose TAA expression and interferon signalling pathways. In addition, tumours frequently evolve to use the programmed cell death 1 (PD-1)/ligand 1 (PD-L1) axis as a means of nullifying attack by immune cells. Evasion of immune recognition or immune escape [24] is now enshrined as a hallmark of cancer [8]. This proliferative signalling is mediated by changes in cytokine signalling (TNF-α, IL-1β, IL-6, IL-10 and TGF-β) [25, 26] and recruitment of suppressive immune cells such as tumour-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) [27] and regulatory T-cells (Tregs) [28, 29] into the TME.
Targeting DNA-Damage Response (DDR) Pathways
Ionising radiation induces lesions in DNA, ranging from simple purine and pyrimidine lesions to single-strand (SSB) and double-strand breaks (DSB) in the DNA [30]. DSB are potentially the most lethal DNA lesions induced by radiotherapy and therapies that can prevent their repair/resolution have the potential to be profoundly radiosensitising. There are specific mechanisms to detect and repair radiation-induced abnormalities in DNA structure: DSBs are repaired by non-homologous end-joining (NHEJ) repair during G1 phase of the cell cycle and by high-fidelity homologous recombination (HR) in S and G2 phases; SSBs and base damage are repaired through the base excision repair (BER) pathway [31].
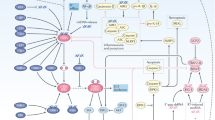
Different types of radiation-induced DNA damage are sensed by mechanisms that activate specific DDR kinases: ataxia telangiectasia-mutated (ATM) and ATR, which phosphorylate the checkpoint kinases, Chk1 and Chk2. In turn, these proteins transfer the signal to different effector molecules that mediate cell cycle arrest, initiate repair functions or trigger cell death – depending on the level of damage sustained by the cell and its capacity to survive and repair that damage. The specific pathways involved are illustrated in simplified form in Fig. 3.1.
A simplified schematic of the DNA damage response. The ATM pathway is activated by DNA double-strand breaks (DSBs), causing activation of Chk2 and p53 with subsequent G1 cell cycle arrest. Diverse inputs converging on RpA-coated single-stranded DNA(ssDNA) activate the ATR-ATRIP complex, with downstream phosphorylation of Chk1, amongst other targets, resulting in G2/M cell cycle arrest arrest. The ATR pathway also plays an important role in S-phase progression and replication fork stabilisation. There is evidence of substantial crosstalk between these pathways (indicated by the dotted arrows). Both ATM and ATR inhibitors (ATMi, ATRi) are in clinical development. (adapted from Dillon et al. Clin Oncol (R Coll Radiol). 2014;26(5):257-65). Key: ATM –ataxia telangiectasia-mutated kinase; ATR –ataxia telangiectasia and Rad3-related kinase; ATRIP –ATR-interacting protein; cdc25A –cell division cycle 25 homolog A; cdc25C –cell division cycle 25 homolog C; cdk1 –cyclin-dependent kinase 1; cdk2 –cyclin-dependent kinase 2; Chk1 –checkpoint kinase 1; Chk2 –checkpoint kinase 2; CyA –cyclin A; CyB –cyclin B; CyE –cyclin E; G1 –gap (or growth) 1 phase of cell cycle; G2 –gap (or growth) 2 phase of cell cycle; IR –ionizing radiation; M –mitosis phase of cell cycle; MRN –Mre11, Rad50 and Nbs1 complex; RpA –replication protein A; S –synthesis phase of cell cycle; UV –ultraviolet light
ATM and ATR Inhibitors
ATM and ATR are key mediators of the DSB signalling response that induce cell cycle arrest to facilitate DNA repair [32]. Conditions that activate ATM and ATR as part of DDR may also participate in regulating the innate immune system and alert it to potentially ‘dangerous’ tumour cells [33].
In response to DSB, the MRE11-RAD50-NBS1 (MRN) complex assembles at DSB sites to act as a DNA damage sensor that activates and recruits ATM to DSB sites [34]. Briefly, when a cell triggers the DDR, ATM initiates a massive signalling cascade with the phosphorylation of hundreds of substrates, including p53 and CHK2 kinase. Activated p53 transactivates the expression of p21Cip1/kip1, which inhibits cyclin-dependent kinase 2 (CDK2) and CDK4/6 to induce G1/S arrest [30]. Inhibition of the ATM/Chk2 axis can lead to replication stress and accumulation of cytosolic DNA that subsequently activates the cGAS-STING-mediated innate immune response [34].
Inhibition of either ATM or ATR has the potential to improve radiotherapy outcomes since they are both key mediators of the DDR [32]. ATM inhibitors such as caffeine [35], wortmannin [36], CP-466722 [37], KU-55933 [38], KU-60019 [39] and KU-59403 [40] increase cell radiosensitivity [41, 42], particularly in p53 low/deficient and PI3K highly-expressing cells [35, 43]. In a preclinical study in vivo with KU60019 and radiotherapy, in addition to tumour cell sensitisation, combination treatment enhanced TBK1 activity, type I interferon production and antigen presentation and increased tumour-infiltrating CD8+ T-cells; moreover, complete responders had established immunological memory with the ability to resist tumour re-challenge [44]. The ATM inhibitor (AZD1390) and radiotherapy is being investigated in a phase I clinical trial in brain cancer (NCT03423628). A dual ATM and DNA-dependent protein kinase (PKc) inhibitor (XRD-0394) is also in clinical development with a phase I trial in combination with radiotherapy recruiting patients (NCT05002140).
ATR is activated by single-stranded DNA (ssDNA) structures that may arise at resected DNA DSBs or stalled replication forks. ATR is recruited via interaction of the regulatory protein ATRIP with ssDNA-bound replication protein A (RPA) [45] (Fig. 3.1). RPA-ssDNA complexes stimulate loading of the RAD9–HUS1–RAD1 (9–1–1) heterotrimer, that recruits TopBP1 which activates ATR [46]. Once ATR is activated, downstream targets, including Chk1, promote DNA repair [47, 48], restart stalled replication forks [49] and intra-S and G2/M cell cycle arrest [50, 51]. In response to DNA damage, activation of the intra-S-phase cell cycle checkpoint slows progression of DNA replication to allow time for resolution [50, 51]. In addition, the ATR-dependent G2/M cell cycle checkpoint is activated through degradation of Cdc25A [51], and phosphorylation of Cdc25C phosphatase inhibits its ability to activate nuclear Cdc2 and, hence, entry into mitosis [52]. Most cancer cells are defective in DNA damage-induced checkpoints, for example through p53 pathway mutations, and this leads to dependence on the intra-S-phase and G2/M checkpoints for cell survival [32]. Therefore, ATR inhibition will lead to accumulation of DNA damage, premature entry into mitosis, mitotic catastrophe and cell death [32].
ATR inhibitors include schisandrin B [53], NU6027 [54], NVP-BEZ235 [55], VE-821 [56], VE-822 [57], AZ20 [58] and AZD6738 [59]. NVP-BEZ235 has been reported to induce marked radiosensitivity in Ras-overexpressing cancers [60], and NU6027 has been shown to increase sensitivity to DNA-damaging agents in breast and ovarian cell lines [54]. VE-822 results in selective sensitisation of pancreatic tumours to radiation in vivo by increasing persistent DNA damage, decreasing cell cycle checkpoint maintenance and reducing homologous recombination repair [57]. In vitro, ATR inhibition downregulates radiotherapy-induced PD-L1/2 expression to sensitise cancer cells to T-cell killing, in addition to potentiating DNA damage [61].
Immune Effects of ATR Inhibition
Promising preclinical in vivo studies of the ATR inhibitor AZD6738 in combination with radiotherapy have shown an enhanced type I/II interferon response and increased immune cell infiltrate [62], increased RT-stimulated CD8+ T-cell infiltration [63, 64], NK-mediated anti-tumour immunity [65], as well as reversal of the Treg immunosuppressive effect [63, 64]. Dillon et al. [62] reported significant radiosensitization to radiotherapy by ceralasertib alongside a marked increase in immune cell infiltration. Increased numbers of CD3+ and NK cells were identified, but the greatest part of the inflammatory infiltrate was composed of myeloid cells. Ceralasertib plus radiation produced a gene expression signature matching a type I/II interferon response with upregulation of genes involved in nucleic acid sensing. Increased major histocompatibility complex class I (MHC-I) levels were observed on tumour cells, with transcript-level data indicating increased antigen processing and presentation within the tumour. Significant modulation of cytokine gene expression (particularly CCL2, CCL5 and CXCL10) was found in vivo, with in vitro data indicating CCL3, CCL5 and CXCL10 are produced from tumour cells after combined therapy with ATR inhibitors (ATRi) and radiation. All of these data point towards opportunities to modulate immune responses triggered by ATRi and radiotherapy through the use of ICPIs that target key regulatory immune checkpoints.
In further studies, Patin et al. [65] evaluated the addition of ICPI (i.e. anti-PD-1, anti-PD-L1, anti-TIGIT) to the ceralasertib and radiotherapy combination, with a view to evaluating if there was further improved response and long-lasting immunity. They showed that ATR inhibition potentiated radiation-mediated tumour control in mouse models of head and neck cancer (MOC2, AT84). ATRi enhanced radiotherapy-induced inflammation in the tumour microenvironment, with NK-cells playing a central role in maximising the effect of treatment. Anti-tumour activity of NK-cells could be further boosted with ICPI targeting TIGIT and PD-L1. In addition, NK-cells were shown to be critical for the induction of T-cell-based immune memory response in mice cured by radiotherapy/ATRi/ICPI combination regimens. Interestingly, analyses of clinical samples from patients receiving ceralasertib confirmed the translational potential of the preclinical studies, including evidence of NK and T-cell activation. Further evaluation of clinical trial material should shed more light on the potential value of ATR inhibitors as adjunctive treatments to immunotherapy-based therapy strategies.
There are, to date, three early phase clinical studies investigating ATR inhibition and radiotherapy. PATRIOT, a phase I study of AZD6738 in combination with palliative radiotherapy, has completed recruitment and is awaiting report (NCT02223923) (Fig. 3.2). BAY1895344 in combination with radiotherapy and pembrolizumab in recurrent head and neck squamous cell carcinoma (HNSCC) (NCT04576091) and M6620 with radiotherapy and chemotherapy in solid cancers (NCT03641547) are also ongoing studies. For at least some of these trials, additional analyses of the immune effects of treatment have the potential to provide further insights into the potential integration of DDR-targeted agents alongside radiation and immunotherapy for the treatment of head and neck (and other) cancers.
Clinical trial design for the PATRIOT study of the ATR inhibitor, ceralasertib(AZD6738). Part A consists of a dose-escalation study of single-agent ceralasertibto define the recommended phase II dose. Part B consists of expansion cohorts using putative markers of replication stress. Part C is a study of ceralasertib at increasing doses and for increasing treatment durations (as indicated by the height and length of the grey boxes labelled “drug”. (adapted from Dillon et al. Clin. Trans. Radiat. Oncol. 2018; 12: 16-20)
Conclusions
In this brief review, we have introduced the concept of targeting the DDR pathway, in this case through ATR inhibition, as a means of sensitising to radiation-induced cell death and triggering anti-tumour immunity. There are, however, a number of clinical challenges that need to be overcome in combining radiotherapy with DDR-targeted agents. These include: (i) the need to define optimal radiation dose-fractionation schedules to be used with DDR inhibitors and ICPIs; (ii) the need to understand how to integrate standard chemoradiation-based therapy regimens into a treatment paradigm based on combining radiation, DDR inhibition and ICPI; and (iii) considerations related to the potentially deleterious effects of wide-field irradiation, for example treatment that encompasses large volumes of tissue containing tumour-draining lymph nodes and large blood vessels (and the circulating immune cells within the bloodstream). In addition, careful attention will need to be paid not just to the acute effects of combination regimens potentially involving radiation, chemotherapy, DDR inhibition and immunotherapy, but also the risks of long-term toxicities. Nonetheless, this is an exciting time for the development of novel treatment strategies that have the potential to change outcomes for patients being treated with curative intent for newly diagnosed locally advanced head and neck cancers.
References
Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104(6):1129–1137.
Verheij M. Clinical biomarkers and imaging for radiotherapy-induced cell death. Cancer Metastasis Rev. 2008;27(3):471–480.
Good JS, Harrington KJ. The hallmarks of cancer and the radiation oncologist: updating the 5Rs of radiobiology. Clin Oncol (R Coll Radiol). 2013;25(10):569–577.
Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045.
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875.
Schaue D, McBride WH. Links between innate immunity and normal tissue radiobiology. Radiat Res. 2010;173(4):406–417.
Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med. 2019;23(8):4854–4865.
Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, Martins I, Schlemmer F, Michaud M, Kepp O, Sukkurwala AQ, Menger L, Vacchelli E, Droin N, Galluzzi L, Krzysiek R, Gordon S, Taylor PR, Van Endert P, Solary E, Smith MJ, Zitvogel L, Kroemer G. Anti-cancer chemotherapy-induced intratumoural recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–741.
Ma Y, Adjemian S, Yang H, Portela Catani JP, Hannani D, Martins I, Michaud M, Kepp O, Sukkurwala AQ, Vacchelli E, Galluzzi L, Zitvogel L, Kroemer G. ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumour bed after anti-cancer chemotherapy. Oncoimmunology. 2013;2(6): e24568.
Kono K, Mimura K. Immunogenic tumour cell death induced by chemoradiotherapy in a clinical setting. Oncoimmunology. 2013;2(1): e22197.
Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumour enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5(2):403–416.
Lhuillier C, Rudqvist N-P, Elemento O, Formenti S, Demaria S. Radiation therapy and anti-tumour immunity: exposing immunogenic mutations to the immune system. Genome Med. 2019;11(1):40.
Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, Breckpot K, Brough D, Chaurio R, Cirone M, Coosemans A, Coulie PG, De Ruysscher D, Dini L, de Witte P, Dudek-Peric AM, Faggioni A, Fucikova J, Gaipl US, Golab J, Gougeon ML, Hamblin MR, Hemminki A, Herrmann M, Hodge JW, Kepp O, Kroemer G, Krysko DV, Land WG, Madeo F, Manfredi AA, Mattarollo SR, Maueroder C, Merendino N, Multhoff G, Pabst T, Ricci JE, Riganti C, Romano E, Rufo N, Smyth MJ, Sonnemann J, Spisek R, Stagg J, Vacchelli E, Vandenabeele P, Vandenberk L, Van den Eynde BJ, Van Gool S, Velotti F, Zitvogel L, Agostinis P. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front Immunol. 2015;6:588.
Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425.
Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–3107.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252.
Wilkins AC, Patin EC, Harrington KJ, Melcher AA. The immunological consequences of radiation-induced DNA damage. J Pathol. 2019;247(5):606–614.
Rudqvist NP, Pilones KA, Lhuillier C, Wennerberg E, Sidhom JW, Emerson RO, Robins HS, Schneck J, Formenti SC, Demaria S. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumour-infiltrating T cells. Cancer Immunol Res. 2018;6(2):139–150.
Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, Yusko EC, Sanders CM, Vignali M, Emerson RO, Robins HS, Wilkinson RW, Honeychurch J, Illidge TM. Fractionated radiation therapy stimulates antitumour immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23(18):5514–5526.
Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377.
Philip M, Rowley DA, Schreiber H. Inflammation as a tumour promoter in cancer induction. Semin Cancer Biol. 2004;14(6):433–439.
Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–1183.
Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol. 2005;26(6):318–325.
Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360.
Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5(6):404–407.
Harris TJ, Hipkiss EL, Borzillary S, Wada S, Grosso JF, Yen HR, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, DeWeese TL, Drake CG. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68(12):1319–1329.
Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G. Functional relationship between tumour-associated macrophages and macrophage colony-stimulating factor as contributors to cancer progression. Front Immunol. 2014;5:489.
Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81(4):1128–1135.
Qu Y, Jin S, Zhang A, Zhang B, Shi X, Wang J, Zhao Y. Gamma-ray resistance of regulatory CD4+CD25+Foxp3+ T cells in mice. Radiat Res. 2010;173(2):148–157.
Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5(1):60.
Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204.
Weber AM, Ryan AJ. ATM and ATR as therapeutic targets in cancer. Pharmacol Ther. 2015;149:124–38.
Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190.
Wang L, Yang L, Wang C, Zhao W, Ju Z, Zhang W, Shen J, Peng Y, An C, Luu YT, Song S, Yap TA, Ajani JA, Mills GB, Shen X, Peng G. Inhibition of the ATM/Chk2 axis promotes cGAS/STING signaling in ARID1A-deficient tumours. J Clin Invest. 2020;130(11):5951–5966.
Powell SN, DeFrank JS, Connell P, Eogan M, Preffer F, Dombkowski D, Tang W, Friend S. Differential sensitivity of p53(-) and p53(+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res. 1995;55(8):1643–1648.
Price BD, Youmell MB. The phosphatidylinositol 3-kinase inhibitor wortmannin sensitizes murine fibroblasts and human tumour cells to radiation and blocks induction of p53 following DNA damage. Cancer Res. 1996;56(2):246–250.
Rainey MD, Charlton ME, Stanton RV, Kastan MB. Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation. Cancer Res. 2008;68(18):7466–7474.
Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64(24):9152–9159.
Golding SE, Rosenberg E, Adams BR, Wignarajah S, Beckta JM, O’Connor MJ, Valerie K. Dynamic inhibition of ATM kinase provides a strategy for glioblastoma multiforme radiosensitization and growth control. Cell Cycle. 2012;11(6):1167–1173.
Batey MA, Zhao Y, Kyle S, Richardson C, Slade A, Martin NM, Lau A, Newell DR, Curtin NJ. Preclinical evaluation of a novel ATM inhibitor, KU59403, in vitro and in vivo in p53 functional and dysfunctional models of human cancer. Mol Cancer Ther. 2013;12(6):959–967.
Vecchio D, Daga A, Carra E, Marubbi D, Raso A, Mascelli S, Nozza P, Garrè ML, Pitto F, Ravetti JL, Vagge S, Corvò R, Profumo A, Baio G, Marcello D, Frosina G. Pharmacokinetics, pharmacodynamics and efficacy on pediatric tumours of the glioma radiosensitizer KU60019. Int J Cancer. 2015;136(6):1445–1457.
Zhang T, Shen Y, Chen Y, Hsieh JT, Kong Z. The ATM inhibitor KU55933 sensitizes radioresistant bladder cancer cells with DAB2IP gene defect. Int J Radiat Biol. 2015;91(4):368–378.
Vecchio D, Daga A, Carra E, Marubbi D, Baio G, Neumaier CE, Vagge S, Corvò R, Pia Brisigotti M, Louis Ravetti J, Zunino A, Poggi A, Mascelli S, Raso A, Frosina G. Predictability, efficacy and safety of radiosensitization of glioblastoma-initiating cells by the ATM inhibitor KU-60019. Int J Cancer. 2014;135(2):479–491.
Zhang Q, Green MD, Lang X, Lazarus J, Parsels JD, Wei S, Parsels LA, Shi J, Ramnath N, Wahl DR, Pasca di Magliano M, Frankel TL, Kryczek I, Lei YL, Lawrence TS, Zou W, Morgan MA. Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy. Cancer Res. 2019; 79(15):3940–3951.
Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92.
Kumagai A, Lee J, Yoo HY, Dunphy WG. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124(5):943–955.
Chen J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 2000;60(18):5037–5039.
Tibbetts RS, Cortez D, Brumbaugh KM, Scully R, Livingston D, Elledge SJ, Abraham RT. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 2000;14(23):2989–3002.
Errico A, Costanzo V. Mechanisms of replication fork protection: a safeguard for genome stability. Crit Rev Biochem Mol Biol. 2012;47(3):222–235.
Sørensen CS, Syljuåsen RG, Falck J, Schroeder T, Rönnstrand L, Khanna KK, Zhou BB, Bartek J, Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3(3):247–258.
Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, Zhang H. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem. 2003;278(24):21767–21773.
Graves PR, Lovly CM, Uy GL, Piwnica-Worms H. Localization of human Cdc25C is regulated both by nuclear export and 14-3-3 protein binding. Oncogene. 2001;20(15):1839–1851.
Nishida H, Tatewaki N, Nakajima Y, Magara T, Ko KM, Hamamori Y, Konishi T. Inhibition of ATR protein kinase activity by schisandrin B in DNA damage response. Nucleic Acids Res. 2009;37(17):5678–5689.
Peasland A, Wang LZ, Rowling E, Kyle S, Chen T, Hopkins A, Cliby WA, Sarkaria J, Beale G, Edmondson RJ, Curtin NJ. Identification and evaluation of a potent novel ATR inhibitor, NU6027, in breast and ovarian cancer cell lines. Br J Cancer. 2011;105(3):372–381.
Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, García-Echeverría C. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumour activity. Mol Cancer Ther. 2008;7(7):1851–1863.
Charrier JD, Durrant SJ, Golec JM, Kay DP, Knegtel RM, MacCormick S, Mortimore M, O’Donnell ME, Pinder JL, Reaper PM, Rutherford AP, Wang PS, Young SC, Pollard JR. Discovery of potent and selective inhibitors of ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase as potential anti-cancer agents. J Med Chem. 2011;54(7):2320–2330.
Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, Vallis KA, Hammond EM, Olcina MM, Gillies McKenna W, Muschel RJ, Brunner TB. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumours to radiation. Cell Death Dis. 2012;3(12):e441.
Foote KM, Blades K, Cronin A, Fillery S, Guichard SS, Hassall L, Hickson I, Jacq X, Jewsbury PJ, McGuire TM, Nissink JW, Odedra R, Page K, Perkins P, Suleman A, Tam K, Thommes P, Broadhurst R, Wood C. Discovery of 4-{4-[(3R)-3-methylmorpholin-4-yl]-6-[1-(methylsulfonyl)cyclopropyl]pyrimidin-2-yl}-1H-indole (AZ20): a potent and selective inhibitor of ATR protein kinase with monotherapy in vivo antitumour activity. J Med Chem. 2013;56(5):2125–2138.
Foote KM, Nissink JWM, McGuire T, Turner P, Guichard S, Yates JWT, Lau A, Blades K, Heathcote D, Odedra R, Wilkinson G, Wilson Z, Wood CM, Jewsbury PJ. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and rad3 related (ATR) kinase with application as an anti-cancer agent. J Med Chem. 2018;61(22):9889–9907.
Konstantinidou G, Bey EA, Rabellino A, Schuster K, Maira MS, Gazdar AF, Amici A, Boothman DA, Scaglioni PP. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69(19):7644–52.
Sun LL, Yang RY, Li CW, Chen MK, Shao B, Hsu JM, Chan LC, Yang Y, Hsu JL, Lai YJ, Hung MC. Inhibition of ATR downregulates PD-L1 and sensitizes tumour cells to T cell-mediated killing. Am J Cancer Res. 2018;8(7):1307–1316.
Dillon MT, Bergerhoff KF, Pedersen M, Whittock H, Crespo-Rodriguez E, Patin EC, Pearson A, Smith HG, Paget JTE, Patel RR, Foo S, Bozhanova G, Ragulan C, Fontana E, Desai K, Wilkins AC, Sadanandam A, Melcher A, McLaughlin M, Harrington KJ. ATR inhibition potentiates the radiation-induced inflammatory tumour microenvironment. Clin Cancer Res. 2019;25(11):3392–3403.
Sheng H, Huang Y, Xiao Y, Zhu Z, Shen M, Zhou P, Guo Z, Wang J, Wang H, Dai W, Zhang W, Sun J, Cao C. ATR inhibitor AZD6738 enhances the antitumour activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumour immune microenvironment in hepatocellular carcinoma. J Immunother Cancer. 2020;8(1):e000340.
Vendetti FP, Karukonda P, Clump DA, Teo T, Lalonde R, Nugent K, Ballew M, Kiesel BF, Beumer JH, Sarkar SN, Conrads TP, O’Connor MJ, Ferris RL, Tran PT, Delgoffe GM, Bakkenist CJ. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumour activity following radiation. J Clin Invest. 2018;128(9):3926–3940.
Patin EC, Dillon MT, Nenclares P, Grove L, Soliman H, Leslie I, Northcote D, Bozhanova G, Crespo-Rodriguez E, Baldock H, Whittock H, Baker G, Kyula J, Guevara J, Melcher AA, Harper J, Ghadially H, Smith S, Pedersen M, McLaughlin M, Harrington KJ. Harnessing radiotherapy-induced NK-cell activity by combining DNA damage-response inhibition and immune checkpoint blockade. J Immunother Cancer. 2022;10(3):e003406.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Harrington, K.J., Hak, C.M.L.C.W., Rullan, A., Patin, E. (2023). DNA Repair Mechanisms as a New Target in Head and Neck Cancer. In: Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, JP., Nicolai, P., O'Sullivan, B. (eds) Critical Issues in Head and Neck Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-23175-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-23175-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23174-2
Online ISBN: 978-3-031-23175-9
eBook Packages: MedicineMedicine (R0)