Abstract
Salivary gland cancer (SGC) encompasses a group of rare malignancies with distinct molecular, histologic, and clinical characteristics. Rarity as well as evolving disease heterogeneity have made treatments particularly challenging, with classic chemotherapy agents showing at best moderate antitumor efficacy in patients with recurrent or metastatic SGC. Recent advances in genomic profiling have provided relevant targets for different subtypes of SGC, enabling tailoring of therapeutic approaches. Despite the lack of predictive biomarkers, treatment with immune checkpoint inhibitors also appears to benefit a subgroup of patients. This chapter aims to give a comprehensive overview of novel approaches to systemic therapy for patients with recurrent or metastatic SGC.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Salivary gland cancer
- Adenoid cystic carcinoma
- Nonadenoid cystic carcinoma
- Targeted therapy
- Immunotherapy
Introduction
Salivary gland cancer (SGC) is a rare malignancy that accounts for less than 5% of all cases of head and neck cancer [1]. The annual age-standardized rate of SGC is 0.57 per 100,000 people worldwide, and is expected to increase by 50% by 2040 [2]. Although the causes remain largely unknown, several factors have been associated with the development of malignant salivary gland tumors, including radiation exposure, history of prior malignancy, viral infections (i.e., Epstein Barr virus [EBV], human immunodeficiency virus [HIV]), industrial chemicals (rubber manufacturing), and nickel compounds [3]. Malignant tumors arising from the major or minor salivary glands are also characterized by considerable diversity. The 5th edition of the World Health Organization (WHO) classification of head and neck tumors identifies 24 distinct histologic subtypes of SGC, with implications to disease biology and clinical features [4].
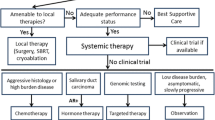
In general, malignant salivary gland tumors have prolonged clinical courses, characterized by slow growth, multiple local recurrences, and delayed development of distant metastases, most commonly to the lung, liver, and bone. Although histologic grading has been shown to possess some prognostic value, implementation of a universal grading scheme appears unable to explain inherent differences in tumor biology and thus, is not currently recommended [5]. Patients with recurrent or metastatic (R/M) disease have poor prognosis and effects of chemotherapy are moderate with a median overall survival (OS) of 15 months and five-year OS rates of about 15% [6]. Moreover, rarity as well as extensive heterogeneity of SGC have precluded the accumulation of prospective clinical trial data, and treatment decisions have been informed by non-randomized studies and/or retrospective series. According to the American Society of Clinical Oncologists (ASCO), systemic therapy should be reserved for patients with metastatic tumor deposits not amenable to palliative local therapy, threatened end-organ dysfunction, or lesions that have grown more than 20% within a period of six months [7].
Recent advances in molecular characterization of SGCs have uncovered subtype-specific genomic alterations with potential clinical significance (Table 20.1) [8]. These approaches have provided deeper understanding of the molecular pathogenesis and are presently included in the definition of several entities, aiding in proper diagnosis [4]. In addition, routine genomic profiling has enabled the development of novel, personalized therapeutic strategies, sometimes via direct extrapolation from progress made in more common tumor types.
Subtypes of Salivary Gland Cancers
Adenoid Cystic Carcinoma
Adenoid cystic carcinoma (ACC) is the most common malignancy arising in the minor salivary glands and second most common overall [9,10,11]. Its clinical course is slow but relentless, marked by perineural invasion, with 40–50% of the patients experiencing disease recurrence after curative intent therapy [11,12,13]. Aside from site of origin and stage, the presence of “any solid” component and/or high-grade transformation/dedifferentiation on histology has been linked with prognosis, with high reproducibility, low interobserver variability, and high negative predictive value [14,15,16,17]. MYB overexpression as well as the presence of activating NOTCH1 mutations have also been shown to confer a poor prognosis for patients with ACC [18,19,20].
ACCs typically harbor a low number of genomic alterations (GA; 1.6 GA/tumor) as well as low TP53 GA frequency (4%) and tumor mutational burden (TMB; TMB >10 mut/Mb in 1% of the cases) [8]. MYB-NFIB fusions, generated by t(6;9)(q22-23;p23-24) translocations, represent the most common GA seen in patients with ACC, occurring in up to 80% of the cases [18, 19, 21]. The product of the MYB gene is a DNA-binding transcription factor that normally regulates stem and progenitor cells [22]. Persson et al. showed that such alterations disrupt repression of MYB resulting in protein overexpression, with implications to apoptosis, cell cycle control, proliferation, cell adhesion, and angiogenesis [23]. Thus, MYB represents a bona fide human oncogene and true hallmark of ACC, irrespective of site of origin [24]. MYB overexpression has been the target of novel vaccination approaches, used synergistically with programmed cell death protein 1 (PD-1) inhibition in the ongoing MYPHISMO trial (NCT03287427) [25]. Additional preclinical efforts to reverse MYB overactivation in patients with ACC have targeted either the upstream IGF1R/AKT signaling axis or the downstream DNA-damage sensor kinase ATR with promising results [26, 27]. MYBL1 rearrangements, including MYBL1-NFIB fusions that may in part be interchangeable with MYB-NFIB, as well as 5’-NFIB fusions that do not involve either the MYB or MYBL1 genes constitute less prevalent events in the genomic landscape of ACC [28].
Notch is a highly evolutionarily conserved pathway that acts as a stem cell fate determinant [29]. There are four NOTCH receptors (NOTCH1-4), and five membrane-bound ligands, including delta-like (DLL1, -3, and -4), and Jagged (JAG1-2); upon interaction, two consecutive proteolytic cleavages of the receptor, the second mediated by the gamma-secretase complex, free the Notch intracellular domain (NICD) and allow it to enter the nucleus and form a transcriptional activation complex that controls the expression of target genes. Activating NOTCH1 mutations have been shown to possess carcinogenic potential, driving 50% of T-cell acute lymphoblastic leukemias (T-ALLs) [30]. They have also been found in 11–29% of patients with ACC [21, 31, 32]. Such patients are more likely to have solid pattern on histology, advanced disease at diagnosis, non-lung metastases, including in liver, bone, or other atypical sites, and ultimately shorter relapse-free survival (RFS) and OS [20]. Several ways to target NOTCH1 have been utilized in patients with ACC. Brontictuzumab is a monoclonal antibody that binds to the negative regulatory region and inhibits NOTCH1; treatment with brontictuzumab led to an objective response rate (ORR) of 17% in the context of a phase I basket study that enrolled 12 patients with ACC, that either harbored a NOTCH1 mutation or were NICD-high on immunohistochemistry (IHC; NCT01778439) [33]. In addition, the ACCURACY phase II clinical trial evaluated the nonspecific gamma secretase inhibitor AL101 in patients with R/M ACC and activating NOTCH1-4 mutations. In cohort 1 (4 mg/week, n = 45), ORR was 15% and disease control rate (DCR) was 65% [34]. In cohort 2 (6 mg/week, n = 42), ORR was 9% and DCR was 70% [35]. The most common toxicity was diarrhea, which was mainly low-grade and tolerable. AL101 is currently being evaluated in the neoadjuvant setting in patients with NOTCH1-driven ACC (NCT04973683). Crenigacestat (LY3039478) represents another gamma secretase inhibitor that has demonstrated clinical activity in heavily pretreated patients with advanced or metastatic disease [36]. Regarding patients with ACC of the salivary gland, crenigacestat showed limited clinical activity with no confirmed responses [37]. Inhibition of cancer stemness kinases by amcasertib (BBI-503) has also demonstrated clinical safety with encouraging signs of antitumor activity, achieving sustained disease control, that warrant its further development in patients with ACC (NCT01781455) [38]. The transcriptional activation complex inhibitor CB-103 has been used to target the Notch pathway in patients with ACC as well (NCT03422679).
Protein arginine methyl-transferase 5 (PRMT5), a highly-conserved metabolic enzyme involved in multiple signal transduction pathways, is also being targeted in patients with ACC. Preliminary results of a phase I study (NCT02783300) demonstrated a partial response in 3 out of 14 patients with ACC that were treated with the PRMT5 inhibitor GSK3326595 [39]. Another PRMT5 inhibitor, PRT543, is currently in development for patients with ACC (NCT03886831).
Overexpression of KIT on IHC has been reported in 65–90% of patients with ACC; however, agents targeting c-KIT, including imatinib and dasatinib, have only achieved sporadic antitumor responses [1, 40]. IHC positivity for epidermal growth factor receptor (EGFR) has also been observed in the majority of ACC cases, but different EGFR-targeted therapies (cetuximab, gefitinib, lapatinib) have failed to produce any antitumor responses [1, 40]. In addition, approximately three out of four ACCs stain positive for vascular endothelial growth factor (VEGF) on IHC [40]. While older multitargeted receptor tyrosine kinase inhibitors (TKI; sunitinib, sorafenib, pazopanib) have shown minor antitumor activity, newer agents have produced more promising results. Specifically, axitinib has been evaluated in the context of two phase II studies (NCT01558661 and NCT02859012) [41, 42]. In the largest study by Keam et al., 60 patients were randomized to axitinib or observation; administration of axitinib resulted in a ORR of 3%, DCR of 100% (versus 52% in the observation arm) and median progression-free survival (PFS) of 10.8 months (versus 2.8 months in the observation arm; hazard ratio [HR], 0.25; 95% confidence intervals [CI], 0.14–0.48; P < 0.0001) [42]. Matters of safety and efficacy for the multitargeted TKI lenvatinib have also been evaluated by two single-arm phase II studies in patients with ACC [43, 44]. The first study (NCT02780310) enrolled 33 patients with R/M ACC and met its primary endpoint of ORR with five partial responses (PR; 16%) [43]. Patients on lenvatinib had a median PFS of 17.5 months. Although there were no new safety signals, 18/32 patients discontinued lenvatinib due to toxicity. In the second study (NCT02860936), 28 patients received lenvatinib with an ORR of 12%, median PFS of 9.1 months and median OS of 27 months; quality of life (QOL) was found to deteriorate in some domains, including fatigue and dry mouth [44]. Recently, the VEGF receptor inhibitor apatinib demonstrated substantial antitumor activity in a single-arm phase II study that enrolled 68 patients with R/M ACC [45]. Patients on apatinib had an ORR and DCR of 46% and 99%, respectively; at a median follow-up of 25.8 months, the median PFS was not reached. As a result, in February 2021, apatinib gained Orphan Drug Designation by the FDA for patients with ACC.
Recently, relevant prostate-specific membrane antigen (PSMA)-ligand uptake has been detected in as high as 93% of ACCs, indicating that therapy with 177Lutetium-PSMA may be beneficial in such patients [46].
Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma (MEC) is the most common malignant salivary gland tumor, accounting for approximately one third of all cases [47]. Most MECs are low-grade tumors and have a good prognosis; five-year cause-specific survival (CSS) rates are 98.8, 97.4, and 67.0% for low-, intermediate-, and high-grade tumors arising in the parotid gland, respectively, and 90.7% for those arising in the minor salivary glands [48, 49]. Age and stage (including nodal status, and the presence of intraparotid or distant metastasis) represent additional prognostic factors for patients with MEC [50, 51]. It should be noted that distant metastases are extremely rare in patients with low-grade MEC (0.2–0.3%) [48, 52].
High-grade tumors typically have more GAs than other-grade tumors (5 GA/tumor for high-grade versus 2.3 and 2.6 GA/tumor for low- and intermediate-grade tumors, respectively; P = 0.019) [53]. Moreover, GAs in TP53, PIK3CA, BRCA1 and BRCA2 are more frequent in high-grade tumors compared with low- or intermediate-grade. Other genomic events frequently reported in patients with MEC, though not grade-specific, include mutations in CDKN2A, CDKN2B, and BAP1. Translocations between mastermind like gene 2 (MAML2) and CREB regulated transcription coactivators (CTRC) represent the most frequent GAs in patients with MEC [54]. CRTC1-MAML2 fusions, most commonly as a result of a t(11;19)(q21;p13) translocation, are seen in the majority of cases, with a higher percentage documented in low- and intermediate-grade tumors; the MAML2 gene occasionally fuses with CRTC3 as well [55, 56]. The clinical significance of these fusions remains to be determined. CRTC1-MAML2 fusion has been shown to upregulate the epidermal growth factor receptor (EGFR) ligand amphiregulin (AREG), causing autocrine EGFR signaling activation, and MEC cell growth and survival [57, 58]. Notably, CRTC1-MAML2-positive MEC tumors are sensitive to EGFR signaling inhibition both in vitro and in vivo in human xenograft models, making it a potential therapeutic target. Although EGFR overexpression on IHC may not be the case in all patients harboring the CRTC1-MAML2 fusion, responses to EGFR inhibitors, such as erlotinib, gefitinib, or cetuximab, have been reported sporadically, making such approaches an attractive option for patients with MEC [40]. However, approaches that used different TKIs, including lapatinib, nintedanib or sorafenib, have failed to demonstrate consistent antitumor activity in patients with MEC [59].
Salivary Duct Carcinoma
Salivary duct carcinoma (SDC) represents 4–10% of all SGCs [40]. Histologically, it is similar to ductal carcinoma of the breast [60]. It typically presents as a rapidly growing mass within the parotid gland; 20–70% of the cases arise from a preexisting pleomorphic adenoma (carcinoma ex pleomorphic adenoma) [60]. SDC is an aggressive tumor with a tendency to metastasize in the lymph nodes (47–68% at presentation) [61, 62]. Notably, 54% of patients treated with curative intent develop locoregional recurrence and/or distant metastasis; brain metastases have been documented in 18% of patients with SDC. The presence of lymph node metastasis as well as the number of involved nodes are independent prognostic factors in patients with SDC [62, 63]. As highlighted by Nakaguro et al., the presence of prominent nuclear pleomorphism, ≥30 mitoses/10 HPF, vascular invasion, or ≥5 poorly differentiated clusters represent additional histologic features associated with poor prognosis [64].
SDCs harbor GAs in ERBB2 in 32% of cases, whereas overexpression of human epidermal growth factor receptor 2 (HER2) by IHC and/or FISH has been recorded in 16–83% [65]. Trastuzumab coupled with docetaxel demonstrated an ORR of 70% in 57 patients with HER2-positive SDC and no prior exposure to HER2 targeted therapy; in this study, HER2 status was assessed with the combination of IHC and FISH and interpreted in accordance with the guidelines for HER2 assessment in breast cancer [66]. Median PFS and OS were 8.9 and 39.7 months, respectively. Addition of pertuzumab to amplify the anti-HER2 activity of first-line treatment regimens has also shown promising results [67]. In a retrospective case series, first line therapy with trastuzumab, pertuzumab and docetaxel achieved an ORRs of 58% (including one complete response [CR]) in patients with HER2-positive SDC (by IHC and/or FISH); ado-trastuzumab emtansine (T-DM1) administered upon disease progression resulted in an ORR of 57% in the same patient population [68]. In addition, median OS reached 42.0 months. As of late, trastuzumab deruxtecan (T-DXd) has also shown promising antitumor activity. In a pooled analysis of two phase I studies (DS8201-A-J101, DS8201-A-A104), encompassing a total of 17 patients, ORR was 47% and DCR was 100%, with a median PFS of 14.1 months [69]. Notably, HER2 status was assessed by either IHC and/or ISH or next generation sequencing (NGS) and 14 patients had previously received treatment with HER2-targeted agents. No new safety signals were recorded—interstitial lung disease was documented in three cases (18%).
Androgen receptor (AR) is expressed in 78–96% of patients with SDC [40, 70]. The first report of androgen-deprivation therapy (ADT; with goserelin) prescribed for the treatment of SDC dates back to 1994 [71]. Combined androgen blockade with leuprorelin and bicalutamide as first-line therapy in patients with R/M SGC was evaluated in a single-arm, single-institution phase II study [72]. Out of 36 patients enrolled, 34 had SDC. AR status was assessed in accordance with the ASCO/College of American Pathologists (CAP) guidelines for the evaluation of breast cancer predictive factors, and tumors were considered positive if a minimum of 1% of tumor nuclei were immunoreactive for AR (AR ≥ 70% was seen in 83% of the cases) [73]. The ORR and DCR were 42% and 86%, respectively. Median PFS was 8.8 months and median OS was 30.5 months. Moreover, ADT with leuprorelin and bicalutamide demonstrated a favorable toxicity profile, with grade 3 or higher adverse events reported in two patients, leading to treatment discontinuation in one. Recently, Locati et al. reported on the efficacy of the combination of abiraterone with a luteinizing hormone-releasing hormone (LHRH) analogue in patients with castration-resistant, AR-expressing SGC (AR ≥ 70% by IHC) [74]. In this phase II study that enrolled 24 patients (19 with SDC), the ORR was 21% and DCR was 63%. In addition, the median PFS was 3.7 months and median OS was 22.5 months. Grade 3 toxicity was reported in 25% of the cases. Moreover, the European Organisation For Research And Treatment Of Cancer Head and Neck Cancer Group/United Kingdom Clinical Research Network (EORTC HNCG/UKCRN) 1206 phase II randomized study has completed accrual and compared ADT with bicalutamide and a gonadotropin-releasing hormone (GnRH) analogue to chemotherapy in previously untreated patients with AR-overexpressing tumors (cohort A; NCT01969578) [75]. Previously treated patients also received ADT as part of study cohort B. ADT has also exhibited clinical efficacy in the adjuvant setting; in a retrospective study, patients with completely resected, stage IVA, AR-positive SDC who received adjuvant ADT demonstrated significantly longer DFS (P = 0.02) and OS (P = 0.03) compared with those who did not [76].
Less frequent mutations seen in patients with SDC may also serve as potential targets for systemic therapy [70, 77]. Indeed, temsirolimus with bevacizumab have been utilized in PIK3CA-mutant tumors (18–27%) [78]. Also, HRAS mutations have been recorded in 16–23% of patients with SDC and treatment with tipifarnib, an inhibitor of farnesyltransferase that ultimately inactivates Ras by preventing it from binding to the membrane, has demonstrated modest antitumor activity with an ORR of 8% and DCR of 62% in 13 previously treated patients with R/M SGC, including 4 with SDC [79]. Finally, the combination of dabrafenib and trametinib has shown clinical activity in a patient harboring BRAF V600E mutation (4–5%) [80].
Mammary Analogue Secretory Carcinoma
Characterized by histological and immunohistochemical resemblance to secretory carcinoma of the breast, mammary analogue secretory carcinoma (MASC) of the salivary gland was first described in 2010 [81]. It is a rare entity that most commonly involves the parotid gland [82]. Although it is marked by histologic diversity, 95–98% of the cases harbor a distinct, recurrent balanced chromosomal translocation t(12;15)(p13;q25), which leads to a fusion gene between the ETS Variant 6 (ETV6) gene on chromosome 12 and the neurotrophic receptor tyrosine kinase (NTRK)3 gene on chromosome 15 and is practically pathognomonic for MASC; the rest 2–5% of the cases harbor rearrangements involving ETV6 and a non-NTRK3 partner [83, 84]. NTRK1-3 encode a family of tropomyosin receptor kinase proteins (TrkA, TrkB, and TrkC, respectively) implicated in the normal development of the nervous system [85]. Fusion of the intact tyrosine kinase domain of NTRK1, NTRK2, or NTRK3 with a variety of partners results in dysregulated activation of several biochemical signaling pathways that promote oncogenesis, including MAPK, PI3K and PKC, in a multitude of solid tumors [86, 87].
The first attempt to target NTRK was made by Drilon et al., who assessed matters of safety and efficacy of the TKR inhibitor larotrectinib, in the context of a phase I-II study in both adults and children with TRK-fusion positive tumors by IHC or FISH, encompassing a total of 17 unique cancer diagnoses [88]. Out of 55 patients enrolled, only one had CNS metastases at baseline. It should be noted that most patients had previously received at least one line of systemic therapy. In 12 patients with MASC, ORR was 83% and median duration of response (DOR) was not reached. In addition, larotrectinib was well tolerated, suggesting that long-term administration would be feasible for patients with TRK-fusion positive disease. Entrectinib is another potent pan-TRK inhibitor with increased antitumor activity in the CNS [89]. In a pooled analysis of three phase I-II studies, entrectinib demonstrated an ORR of 83% in patients with MASC of the salivary gland; out of 24 patients enrolled, 20 responded to entrectinib. Interestingly, ORR was highest among patients with MASC of the salivary gland compared with other tumor types. Again, this study enrolled mostly previously treated patients (63%). Patients with CNS metastases represented 21% of the study cohort; intracranial ORR with entrectinib was 53% in patients with measurable disease in the CNS and none of the patients without CNS metastases had confirmed progression in the CNS at data cutoff, achieving a 12-month event-free rate of 100%. PBI-200, a next-generation TRK kinase inhibitor that demonstrates clinical activity against relevant resistance mutations after treatment with a first-generation agent as well as enhanced brain penetration is currently being evaluated in the PBI-200-101 phase I/II trial in patients with NTRK-fusion-positive advanced solid tumors (NCT04901806).
Acinic Cell Carcinoma
Acinic cell carcinoma is responsible for approximately 10% of all SGCs [47, 90]. This subtype most commonly arises in the major salivary glands [91]. It is a low-grade tumor, that is predominantly composed of acinic serous cells with zymogen-secreting granules and has a relatively slow growth pattern [92, 93]. Male sex, age >45 years, and tumor size >3 cm represent factors independently associated with prognosis [94]. The presence of aberrations in MSANTD3 gene, most commonly HTN3-MSANTD3 fusions, is highly specific for acinic cell carcinoma, characterizing 4–16% of the cases [95, 96]. However, their oncogenic potential as well as therapeutic relevance remain questionable. Nevertheless, NTRK gene fusion analysis is advised for all patients diagnosed with acinic cell carcinoma as MASC was formerly classified with the latter and has only been described as a separate entity since 2010.
Polymorphous Adenocarcinoma
Consisting of tumors previously classified as polymorphous low-grade adenocarcinoma (PLGA) or cribriform adenocarcinoma of the minor salivary gland (CAMSG), polymorphous adenocarcinoma (PAC) is characterized by cytologic uniformity but architectural diversity [97, 98]. PAC is the second most common malignancy of the minor salivary glands [98]. Overall, it is an indolent disease that rarely presents with distant metastases (4.3%) and has a good prognosis with 10-year DSS rates of 94% [99, 100]. PACs typically harbor GAs that affect the PKRD genes; the PRKD1 E710D hotspot mutation is present in >70% of PLGA cases, whereas 80% of CAMSGs display rearrangements involving PRKD1, PRKD2, or PRKD3 (PRKD1/2/3); although these GAs appear mutually exclusive, PACs have marked genetic overlap, essentially representing a spectrum of lesions driven by GAs in the PKRD genes [101, 102]. Fusion-positive tumors are usually spotted at the base of the tongue, show papillary architecture, and have an increased risk of nodal metastasis [103]. Non-targetable GAs affecting the FGFR1 gene have also been documented in 20% of all PLGA tumors [8].
Adenocarcinoma not Otherwise Specified (NOS)
By definition, adenocarcinoma NOS represents a residual group of salivary gland malignancies that cannot be classified into one of the other subtypes. The reported rates of adenocarcinoma NOS range between 1.8 and 12.2% [47, 90]. However, these rates may overestimate its actual prevalence due to misclassification, and advances in molecular characterization of SGC are expected to curtail this remaining group. Similar to SDC, these tumors have a relatively increased load of GAs (4.1 GA/tumor), with GAs in TP53 observed in 55% of the cases [8]. Although the rates of either HER2 or AR positivity are lower compared with SDC, it is reasonable to test all adenocarcinomas NOS for both targets and treat accordingly. Indeed, T-DM1 has demonstrated enhanced antitumor efficacy in HER2-amplified adenocarcinoma NOS [104, 105]. In addition, patients with adenocarcinoma NOS have been included in trials evaluating ADT in SGC, however results of this subgroup were not reported separately [72, 74]. Additional GAs that have been described in adenocarcinoma NOS involve the PI3K-pathway, cyclin dependent kinases, and RAS family of proteins, as mentioned above [106].
Carcinoma ex Pleomorphic Adenoma
Carcinoma ex pleomorphic adenoma accounts for 8–12% of all SGCs [47, 90, 107]. It arises within a preexisting polymorphous adenoma (PA) and primarily affects the major salivary glands, most commonly the parotid [107]. The extent of tumor invasion through the PA capsule into the surrounding tissue has been found to correlate with disease outcome [108, 109]. Gene fusions involving PLAG1 and less frequently HMGA2 have been documented in up to 86% of the cases [110]. Although of diagnostic importance, the clinical utility of these rearrangements has not been clearly delineated. As far as systemic therapy is concerned, adequate description of the subtype of the carcinoma component is crucial for optimizing therapeutic strategy; SDC represents the most common histologic subtype, followed by myoepithelial carcinoma [110].
Other Subtypes
Other subtypes of SGC are very rare, not characterized by targetable GAs or seldom require systemic therapy due to low rates of recurrence and/or metastasis. These are listed in Table 20.1.
Immunotherapy
Inhibition of the PD-1/ligand (PD-L1) immune checkpoint has achieved clinical responses in 15–20% of patients with R/M squamous cell carcinoma of the head and neck (SCCHN) [111,112,113]. However, data regarding the efficacy of immune checkpoint blockade in patients with SGC are scarce. Overall, SGCs have low PD-L1 expression. The KEYNOTE-028 phase Ib basket trial enrolled a cohort of 26 patients with PD-L1-positive (PD-L1 combined positive score [CPS] ≥1) R/M SGC [114]. Pembrolizumab showed modest clinical activity with an ORR of 12% and DCR of 58%. The median PFS and OS reached 3.8 and 13.0 months, respectively. Antitumor efficacy was only slightly better with the addition of vorinostat [115]. In addition, single-agent PD-1 blockade with nivolumab achieved an ORR of 9% in unselected patients with ACC versus 4% in those with non-ACC histology, with a median PFS of 4.9 and 1.8 months, respectively [116].
The TMB is significantly lower in SGCs compared with tumor types where immunotherapy is currently approved (i.e., non-small cell lung cancer [NSCLC], melanoma). Specifically, less than 5% of clinically indolent tumors, such as ACC, acinic cell carcinoma, and MASC, harbor >10 mut/Mb, whereas for more aggressive subtypes, such as MEC, SDC, and adenocarcinoma NOS, the relative frequency does not exceed 15% [8]. Moreover, SDCs appear immune infiltrated and express immune checkpoints in abundancy in contrast to ACCs that have an immune-depleted tumor microenvironment, characterized by the presence of M2-polarized macrophages and myeloid-derived suppressor cells [117]. In line with the above, combination immunotherapy with nivolumab plus ipilimumab achieved an ORR of 6% (2/32) in patients with R/M ACC, compared with 16% (5/32) in those with non-ACC, in the context of a phase II trial (NCT0317624); importantly, responses were deep, durable and more common in patients with SDC [118, 119]. Similar results were reported in the SWOG S1609—DART trial, where patients with ACC histology had an ORR of 4% and patients with non-ACC histology had an ORR of 9% [120]. Ongoing clinical trials evaluating immune checkpoint inhibitors in patients with SGC are presented in Table 20.2.
Conclusions
Both rarity and diversity of SGC pose challenges in conducting prospective clinical trials. As a result, we are lacking phase III data and quality of evidence that supports current clinical practice guidelines is low [7]. Recent advances in the molecular characterization of these tumors have unveiled multiple, oftentimes targetable, subtype-specific alterations. Thus, adequate pathological diagnosis by an expert salivary gland pathologist to determine the exact subtype of SGC is key in choosing the right systemic therapy. Participation in a clinical trial should be encouraged in all patients with SGC. For patients with ACC, a multitargeted tyrosine kinase inhibitor (i.e., lenvatinib, apatinib) may be offered. For those with non-ACC histology, therapy should be tailored to tumor molecular alterations (i.e., AR, HER2, NTRK). Next generation sequencing has to be considered for patients with SGC as it may offer the potential for targeted therapies. As far as immunotherapy is concerned, it should not be offered routinely, but may have a role in select patients, either in the context of a clinical trial or under local regulatory approval. The study of novel agents in prospective multicenter clinical trials, preferably in biomarker-selected populations, will be pivotal for the development of evidence-based approaches in SGC.
References
Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24(17):2673–8.
Global Cancer Observatory [Internet]. International Agency for Research on Cancer/World Health Organization; updated 2022. https://gco.iarc.fr.
Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. 2010;74(2):134–48.
Board WCoTE. Head and neck tumours. Lyon: IARC Press; 2022.
Seethala RR. Histologic grading and prognostic biomarkers in salivary gland carcinomas. Adv Anat Pathol. 2011;18(1):29–45.
Nam SJ, Roh JL, Cho KJ, Choi SH, Nam SY, Kim SY. Risk factors and survival associated with distant metastasis in patients with carcinoma of the salivary gland. Ann Surg Oncol. 2016;23(13):4376–83.
Geiger JL, Ismaila N, Beadle B, Caudell JJ, Chau N, Deschler D, et al. Management of salivary gland malignancy: ASCO guideline. J Clin Oncol. 2021;39(17):1909–41.
Ross JS, Gay LM, Wang K, Vergilio JA, Suh J, Ramkissoon S, et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann Oncol. 2017;28(10):2539–46.
Perzin KH, Gullane P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978;42(1):265–82.
Chomette G, Auriol M, Tranbaloc P, Vaillant JM. Adenoid cystic carcinoma of minor salivary glands. Analysis of 86 cases. Clinico-pathological, histoenzymological and ultrastructural studies. Virchows Arch A Pathol Anat Histol. 1982;395(3):289–301.
Bradley PJ. Adenoid cystic carcinoma evaluation and management: progress with optimism! Curr Opin Otolaryngol Head Neck Surg. 2017;25(2):147–53.
Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125(2):149–52.
Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174(5):495–8.
Ciccolallo L, Licitra L, Cantú G, Gatta G. Survival from salivary glands adenoid cystic carcinoma in European populations. Oral Oncol. 2009;45(8):669–74.
van Weert S, van der Waal I, Witte BI, Leemans CR, Bloemena E. Histopathological grading of adenoid cystic carcinoma of the head and neck: analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 2015;51(1):71–6.
Atallah S, Casiraghi O, Fakhry N, Wassef M, Uro-Coste E, Espitalier F, et al. A prospective multicentre REFCOR study of 470 cases of head and neck Adenoid cystic carcinoma: epidemiology and prognostic factors. Eur J Cancer. 2020;130:241–9.
Skalova A, Leivo I, Hellquist H, Agaimy A, Simpson RHW, Stenman G, et al. High-grade transformation/dedifferentiation in salivary gland carcinomas: Occurrence across subtypes and clinical significance. Adv Anat Pathol. 2021;28(3):107–18.
Mitani Y, Li J, Rao PH, Zhao YJ, Bell D, Lippman SM, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16(19):4722–31.
Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao YJ, et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17(22):7003–14.
Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P, et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Oncol. 2017;35(3):352–60.
Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45(7):791–8.
Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8(7):523–34.
Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106(44):18740–4.
Gonda TJ, Ramsay RG. Adenoid cystic carcinoma can be driven by MYB or MYBL1 rearrangements: New insights into MYB and tumor biology. Cancer Discov. 2016;6(2):125–7.
Pham T, Pereira L, Roth S, Galletta L, Link E, Akhurst T, et al. First-in-human phase I clinical trial of a combined immune modulatory approach using TetMYB vaccine and Anti-PD-1 antibody in patients with advanced solid cancer including colorectal or adenoid cystic carcinoma: The MYPHISMO study protocol (NCT03287427). Contemp Clin Trials Commun. 2019;16: 100409.
Andersson MK, Afshari MK, Andrén Y, Wick MJ, Stenman G. Targeting the oncogenic transcriptional regulator MYB in adenoid cystic carcinoma by inhibition of IGF1R/AKT signaling. J Natl Cancer Inst. 2017;109(9).
Andersson MK, Mangiapane G, Nevado PT, Tsakaneli A, Carlsson T, Corda G, et al. ATR is a MYB regulated gene and potential therapeutic target in adenoid cystic carcinoma. Oncogenesis. 2020;9(1):5.
Mitani Y, Liu B, Rao PH, Borra VJ, Zafereo M, Weber RS, et al. Novel MYBL1 gene rearrangements with recurrent MYBL1-NFIB fusions in salivary adenoid cystic carcinomas lacking t(6;9) translocations. Clin Cancer Res. 2016;22(3):725–33.
Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011;208(10):1931–5.
Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–71.
Stephens PJ, Davies HR, Mitani Y, Van Loo P, Shlien A, Tarpey PS, et al. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. 2013;123(7):2965–8.
Ross JS, Wang K, Rand JV, Sheehan CE, Jennings TA, Al-Rohil RN, et al. Comprehensive genomic profiling of relapsed and metastatic adenoid cystic carcinomas by next-generation sequencing reveals potential new routes to targeted therapies. Am J Surg Pathol. 2014;38(2):235–8.
Ferrarotto R, Eckhardt G, Patnaik A, LoRusso P, Faoro L, Heymach JV, et al. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann Oncol. 2018;29(7):1561–8.
Ferrarotto R, Wirth LJ, Muzaffar J, Rodriguez CP, Xia B, Perez CA, Bowles DW, Winquist E, Hotte SJ, Metcalf R, Even C, Gordon GB, Gordon G, Ho A. 919MO—ACCURACY a phase II trial of AL101, a selective gamma secretase inhibitor, in subjects with recurrent/metastatic (R/M) adenoid cystic carcinoma (ACC) harboring Notch activating mutations (Notchmut). Ann Oncol. 2020;S599–S628.
Ho AL, Bowles DW, Even C, Hao D, Kang H, Metcalf R, Muzaffar J, Oliva M, Perez CA, Popovtzer A, Rodriguez CP, Stemmer SM, Van Herpen CM, Winquist E, Wirth LJ, Worden FP, Xia B, Gordon G, Gordon GB, Ferrarotto R. 904P—ACCURACY: A phase II trial of AL101, a selective gamma secretase inhibitor, in subjects with recurrent/metastatic (R/M) adenoid cystic carcinoma (ACC) harboring Notch activating mutations (Notchmut): Results of 6-mg cohort. Ann Oncol. 2021;S786–S817.
Massard C, Azaro A, Soria JC, Lassen U, Le Tourneau C, Sarker D, et al. First-in-human study of LY3039478, an oral Notch signaling inhibitor in advanced or metastatic cancer. Ann Oncol. 2018;29(9):1911–7.
Even C, Lassen U, Merchan J, Le Tourneau C, Soria JC, Ferte C, et al. Safety and clinical activity of the Notch inhibitor, crenigacestat (LY3039478), in an open-label phase I trial expansion cohort of advanced or metastatic adenoid cystic carcinoma. Invest New Drugs. 2020;38(2):402–9.
Cote GM, Edenfield WJ, Laurie SA, Chau NG, Becerra C, Spira AI, et al. A phase 1b/2 study of amcasertib, a first-in-class cancer stemness kinase inhibitor, in advanced adenoid cystic carcinoma. J Clin Oncol. 2017;35(15_suppl):6036–6036.
Siu L. 3410—METEOR-1: a phase i study of GSK3326595, a first-in-class protein arginine methyltransferase 5 (PRMT5) inhibitor, in advanced solid tumors. Ann Oncol. 2019;30.
Lassche G, van Boxtel W, Ligtenberg MJL, van Engen-van Grunsven ACH, van Herpen CML. Advances and challenges in precision medicine in salivary gland cancer. Cancer Treat Rev. 2019;80: 101906.
Ho AL, Dunn L, Sherman EJ, Fury MG, Baxi SS, Chandramohan R, et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann Oncol. 2016;27(10):1902–8.
Keam B, Kang EJ, Ahn M-J, Ock C-Y, Lee KW, Kwon JH, et al. Randomized phase II study of axitinib versus observation in patients with recurred or metastatic adenoid cystic carcinoma. J Clin Oncol. 2020;38(15_suppl):6503–6503.
Tchekmedyian V, Sherman EJ, Dunn L, Tran C, Baxi S, Katabi N, et al. Phase II study of lenvatinib in patients with progressive, recurrent or metastatic adenoid cystic carcinoma. J Clin Oncol. 2019;37(18):1529–37.
Locati LD, Galbiati D, Calareso G, Alfieri S, Singer S, Cavalieri S, et al. Patients with adenoid cystic carcinomas of the salivary glands treated with lenvatinib: activity and quality of life. Cancer. 2020;126(9):1888–94.
Zhu G, Zhang L, Dou S, Li R, Li J, Ye L, et al. Apatinib in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: a single-arm, phase II prospective study. Ther Adv Med Oncol. 2021;13:17588359211013626.
van Boxtel W, Lütje S, van Engen-van Grunsven ICH, Verhaegh GW, Schalken JA, Jonker MA, et al. (68)Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: a phase 2 imaging study. Theranostics. 2020;10(5):2273–83.
Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. 2008;44(4):407–17.
Chen MM, Roman SA, Sosa JA, Judson BL. Histologic grade as prognostic indicator for mucoepidermoid carcinoma: a population-level analysis of 2400 patients. Head Neck. 2014;36(2):158–63.
Cheraghlou S, Kuo P, Mehra S, Agogo GO, Bhatia A, Husain ZA, et al. Adjuvant therapy in major salivary gland cancers: analysis of 8580 patients in the national cancer database. Head Neck. 2018;40(7):1343–55.
Cheng EY, Kim JH, Grose EM, Philteos J, Levin M, de Almeida J, et al. Clinicopathological predictors of survival for parotid mucoepidermoid carcinoma: a systematic review. Otolaryngol Head Neck Surg. 2022:1945998221086845.
Mimica X, Yuan A, Hay A, Katabi N, Zanoni DK, Valero C, et al. Mucoepidermoid carcinoma: evaluating the prognostic impact of primary tumor site. Oral Oncol. 2021;123: 105602.
Benchetrit L, Mehra S, Mahajan A, Rahmati RW, Judson BL, Edwards HA. Major salivary gland cancer with distant metastasis upon presentation: patterns, outcomes, and imaging implications. Otolaryngol Head Neck Surg. 2021:1945998211058354.
Wang K, McDermott JD, Schrock AB, Elvin JA, Gay L, Karam SD, et al. Comprehensive genomic profiling of salivary mucoepidermoid carcinomas reveals frequent BAP1, PIK3CA, and other actionable genomic alterations. Ann Oncol. 2017;28(4):748–53.
Nordkvist A, Gustafsson H, Juberg-Ode M, Stenman G. Recurrent rearrangements of 11q14-22 in mucoepidermoid carcinoma. Cancer Genet Cytogenet. 1994;74(2):77–83.
Kang H, Tan M, Bishop JA, Jones S, Sausen M, Ha PK, et al. Whole-exome sequencing of salivary gland mucoepidermoid carcinoma. Clin Cancer Res. 2017;23(1):283–8.
Jee KJ, Persson M, Heikinheimo K, Passador-Santos F, Aro K, Knuutila S, et al. Genomic profiles and CRTC1-MAML2 fusion distinguish different subtypes of mucoepidermoid carcinoma. Mod Pathol. 2013;26(2):213–22.
Chen Z, Chen J, Gu Y, Hu C, Li JL, Lin S, et al. Aberrantly activated AREG-EGFR signaling is required for the growth and survival of CRTC1-MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene. 2014;33(29):3869–77.
Coxon A, Rozenblum E, Park YS, Joshi N, Tsurutani J, Dennis PA, et al. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res. 2005;65(16):7137–44.
Sama S, Komiya T, Guddati AK. Advances in the treatment of mucoepidermoid carcinoma. World J Oncol. 2022;13(1):1–7.
Schmitt NC, Kang H, Sharma A. Salivary duct carcinoma: an aggressive salivary gland malignancy with opportunities for targeted therapy. Oral Oncol. 2017;74:40–8.
Xiao CC, Zhan KY, White-Gilbertson SJ, Day TA. Predictors of nodal metastasis in parotid malignancies: a national cancer data base study of 22,653 patients. Otolaryngol Head Neck Surg. 2016;154(1):121–30.
Boon E, Bel M, van Boxtel W, van der Graaf WTA, van Es RJJ, Eerenstein SEJ, et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from The Netherlands. Int J Cancer. 2018;143(4):758–66.
Otsuka K, Imanishi Y, Tada Y, Kawakita D, Kano S, Tsukahara K, et al. Clinical outcomes and prognostic factors for salivary duct carcinoma: a multi-institutional analysis of 141 patients. Ann Surg Oncol. 2016;23(6):2038–45.
Nakaguro M, Sato Y, Tada Y, Kawakita D, Hirai H, Urano M, et al. Prognostic implication of histopathologic indicators in salivary duct carcinoma: proposal of a novel histologic risk stratification model. Am J Surg Pathol. 2020;44(4):526–35.
Nakaguro M, Tada Y, Faquin WC, Sadow PM, Wirth LJ, Nagao T. Salivary duct carcinoma: updates in histology, cytology, molecular biology, and treatment. Cancer Cytopathol. 2020;128(10):693–703.
Takahashi H, Tada Y, Saotome T, Akazawa K, Ojiri H, Fushimi C, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J Clin Oncol. 2019;37(2):125–34.
Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from mypathway, an open-label, phase iia multiple basket study. J Clin Oncol. 2018;36(6):536–42.
Uijen MJM, Lassche G, van Engen-van Grunsven ACH, Driessen CML, van Herpen CML. Case series of docetaxel, trastuzumab, and pertuzumab (DTP) as first line anti-HER2 therapy and ado-trastuzumab emtansine (T-DM1) as second line for recurrent or metastatic HER2-positive salivary duct carcinoma. Oral Oncol. 2022;125: 105703.
Bando H, Kinoshita I, Modi S, Tsurutani J, Bang Y-J, Iwata H, et al. Trastuzumab deruxtecan (T-DXd) in patients with human epidermal growth factor receptor 2 (HER2)-expressing salivary duct carcinoma: Subgroup analysis of two phase 1 studies. J Clin Oncol. 2021;39(15_suppl):6079–6079.
Takase S, Kano S, Tada Y, Kawakita D, Shimura T, Hirai H, et al. Biomarker immunoprofile in salivary duct carcinomas: clinicopathological and prognostic implications with evaluation of the revised classification. Oncotarget. 2017;8(35):59023–35.
van der Hulst RW, van Krieken JH, van der Kwast TH, Gerritsen JJ, Baatenburg de Jong RJ, Lycklama à Nijeholt AA, et al. Partial remission of parotid gland carcinoma after goserelin. Lancet. 1994;344(8925):817.
Fushimi C, Tada Y, Takahashi H, Nagao T, Ojiri H, Masubuchi T, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979–84.
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–95.
Locati LD, Cavalieri S, Bergamini C, Resteghini C, Colombo E, Calareso G, et al. Abiraterone acetate in patients with castration-resistant, androgen receptor-expressing salivary gland cancer: a phase II trial. J Clin Oncol. 2021;39(36):4061–8.
Locati LD, Caballero C, Fortpied C, Perrone F, Pilotti S, Harrington KJ, et al. Hope for salivary gland cancer (SGC): EORTC HNCG/UKCRN 1206 randomized phase II study to evaluate the efficacy and safety of Chemotherapy (CT) vs androgen deprivation therapy (ADT) in patients with recurrent and/or metastatic androgen receptor (AR) expressing SGC. Ann Oncol. 2017;28:v392–3.
van Boxtel W, Locati LD, van Engen-van Grunsven ACH, Bergamini C, Jonker MA, Fiets E, et al. Adjuvant androgen deprivation therapy for poor-risk, androgen receptor-positive salivary duct carcinoma. Eur J Cancer. 2019;110:62–70.
Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22(18):4623–33.
Piha-Paul SA, Cohen PR, Kurzrock R. Salivary duct carcinoma: targeting the phosphatidylinositol 3-kinase pathway by blocking mammalian target of rapamycin with temsirolimus. J Clin Oncol. 2011;29(26):e727–30.
Hanna GJ, Guenette JP, Chau NG, Sayehli CM, Wilhelm C, Metcalf R, et al. Tipifarnib in recurrent, metastatic HRAS-mutant salivary gland cancer. Cancer. 2020;126(17):3972–81.
Lin VTG, Nabell LM, Spencer SA, Carroll WR, Harada S, Yang ES. First-line treatment of widely metastatic BRAF-mutated salivary duct carcinoma with combined BRAF and MEK inhibition. J Natl Compr Canc Netw. 2018;16(10):1166–70.
Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608.
Khalele BA. Systematic review of mammary analog secretory carcinoma of salivary glands at 7 years after description. Head Neck. 2017;39(6):1243–8.
Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol. 2016;27(5):920–6.
Skálová A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard rt-pcr: report of 4 cases harboring ETV6-X gene fusion. Am J Surg Pathol. 2016;40(1):3–13.
Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–47.
Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5(1):25–34.
Vaishnavi A, Capelletti M, Le AT, Kako S, Butaney M, Ercan D, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19(11):1469–72.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9.
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–82.
Bjørndal K, Krogdahl A, Therkildsen MH, Overgaard J, Johansen J, Kristensen CA, et al. Salivary gland carcinoma in Denmark 1990–2005: a national study of incidence, site and histology. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol. 2011;47(7):677–82.
Biron VL, Lentsch EJ, Gerry DR, Bewley AF. Factors influencing survival in acinic cell carcinoma: a retrospective survival analysis of 2061 patients. Head Neck. 2015;37(6):870–7.
Perzin KH, LiVolsi VA. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979;44(4):1434–57.
Batsakis JG, Luna MA, el-Naggar AK. Histopathologic grading of salivary gland neoplasms: II. Acinic cell carcinomas. Ann Otol Rhinol Laryngol. 1990;99(11):929–33.
Neskey DM, Klein JD, Hicks S, Garden AS, Bell DM, El-Naggar AK, et al. Prognostic factors associated with decreased survival in patients with acinic cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1195–202.
Andreasen S, Varma S, Barasch N, Thompson LDR, Miettinen M, Rooper L, et al. The HTN3-MSANTD3 fusion gene defines a subset of acinic cell carcinoma of the salivary gland. Am J Surg Pathol. 2019;43(4):489–96.
Barasch N, Gong X, Kwei KA, Varma S, Biscocho J, Qu K, et al. Recurrent rearrangements of the Myb/SANT-like DNA-binding domain containing 3 gene (MSANTD3) in salivary gland acinic cell carcinoma. PLoS ONE. 2017;12(2): e0171265.
Hernandez-Prera JC. Historical evolution of the polymorphous adenocarcinoma. Head Neck Pathol. 2019;13(3):415–22.
Katabi N, Xu B. Polymorphous adenocarcinoma. Surg Pathol Clin. 2021;14(1):127–36.
Mimica X, Katabi N, McGill MR, Hay A, Zanoni DK, Shah JP, et al. Polymorphous adenocarcinoma of salivary glands. Oral Oncol. 2019;95:52–8.
Patel TD, Vazquez A, Marchiano E, Park RC, Baredes S, Eloy JA. Polymorphous low-grade adenocarcinoma of the head and neck: a population-based study of 460 cases. Laryngoscope. 2015;125(7):1644–9.
Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–9.
Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer. 2014;53(10):845–56.
Sebastiao APM, Xu B, Lozada JR, Pareja F, Geyer FC, Da Cruz PA, et al. Histologic spectrum of polymorphous adenocarcinoma of the salivary gland harbor genetic alterations affecting PRKD genes. Mod Pathol. 2020;33(1):65–73.
Li BT, Shen R, Offin M, Buonocore DJ, Myers ML, Venkatesh A, et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): results from a phase II basket trial. J Clin Oncol. 2019;37(15_suppl):6001–6001.
Jhaveri KL, Wang XV, Makker V, Luoh SW, Mitchell EP, Zwiebel JA, et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol. 2019;30(11):1821–30.
Wang K, Russell JS, McDermott JD, Elvin JA, Khaira D, Johnson A, et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin Cancer Res. 2016;22(24):6061–8.
Antony J, Gopalan V, Smith RA, Lam AK. Carcinoma ex pleomorphic adenoma: a comprehensive review of clinical, pathological and molecular data. Head Neck Pathol. 2012;6(1):1–9.
Hu YH, Li W, Zhang CY, Xia RH, Tian Z, Wang LZ, et al. Prognostic nomogram for disease-specific survival of carcinoma ex pleomorphic adenoma of the salivary gland. Head Neck. 2017;39(12):2416–24.
Katabi N, Gomez D, Klimstra DS, Carlson DL, Lee N, Ghossein R. Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: a clinicopathologic study of 43 cases. Hum Pathol. 2010;41(7):927–34.
Katabi N, Ghossein R, Ho A, Dogan S, Zhang L, Sung YS, et al. Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum Pathol. 2015;46(1):26–33.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67.
Ferris R, Gillison ML. Nivolumab for squamous-cell cancer of head and neck. N Engl J Med. 2017;376(6):596.
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–28.
Cohen RB, Delord JP, Doi T, Piha-Paul SA, Liu SV, Gilbert J, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 Study. Am J Clin Oncol. 2018;41(11):1083–8.
Rodriguez CP, Wu QV, Voutsinas J, Fromm JR, Jiang X, Pillarisetty VG, et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin Cancer Res. 2020;26(4):837–45.
Fayette J, Even C, Digue L, Geoffrois L, Rolland F, Cupissol D, et al. NISCAHN: A phase II, multicenter nonrandomized trial aiming at evaluating nivolumab (N) in two cohorts of patients (pts) with recurrent/metastatic (R/M) salivary gland carcinoma of the head and neck (SGCHN), on behalf of the Unicancer Head & Neck Group. J Clin Oncol. 2019;37(15_suppl):6083–6083.
Linxweiler M, Kuo F, Katabi N, Lee M, Nadeem Z, Dalin MG, et al. The immune microenvironment and neoantigen landscape of aggressive salivary gland carcinomas differ by subtype. Clin Cancer Res. 2020;26(12):2859–70.
Tchekmedyian V, Sherman EJ, Dunn L, Fetten JV, Michel LS, Kriplani A, et al. A phase II trial cohort of nivolumab plus ipilimumab in patients (Pts) with recurrent/metastatic adenoid cystic carcinoma (R/M ACC). J Clin Oncol. 2019;37(15_suppl):6084–6084.
Burman B, Sherman EJ, Dunn L, Fetten JV, Michel LS, Morris LGT, et al. A phase II trial cohort of nivolumab plus ipilimumab in patients (Pts) with recurrent/metastatic salivary gland cancers (R/M SGCs). J Clin Oncol. 2021;39(15_suppl):6002–6002.
Chae YK, Othus M, Patel SP, Ohr JP, Worden FP, Suga JM, et al. Abstract 3418: a phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) SWOG S1609: the salivary gland tumor cohort. Cancer Res. 2020;80(16_Supplement):Abstract 3418.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Vathiotis, I.A., Johnson, J.M., Argiris, A. (2023). New Systemic Therapies in Salivary Gland Cancer. In: Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, JP., Nicolai, P., O'Sullivan, B. (eds) Critical Issues in Head and Neck Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-23175-9_20
Download citation
DOI: https://doi.org/10.1007/978-3-031-23175-9_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23174-2
Online ISBN: 978-3-031-23175-9
eBook Packages: MedicineMedicine (R0)