Abstract
Immune checkpoint inhibition has emerged as an integral part of the standard-of-care for head and neck squamous cell carcinoma (HNSCC) in recurrent and/or metastatic stages. Clinical responses are impressive but remain limited to a minority of patients. Primary resistance of never-responders is considered to derive from host- and tumor-specific characteristics, the latter comprising tumor immune checkpoint activity, immune contexture, tumor mutational burden, neo-antigen load, and others. Secondary resistance of initially responding patients in addition, appears to be driven predominantly by irreversible T-cell exhaustion and therapy-induced selection of tumor cell clones with mutations in critical genes involved in the response to immune checkpoint inhibition. With particular focus on primary resistance against immune checkpoint inhibition, scientific interest of preclinical and clinical researchers currently aims at the development and evaluation of combined modality treatment approaches. Radiotherapy is a highly promising partner in this regard and represents a crucial treatment modality for patients with locally advanced HNSCC. Historically established as cytotoxic anti-cancer treatment, a growing body of evidence has shown additional locoregional and systemic immunomodulatory effects of radiotherapy. These are largely attributed to reprogramming of the tumor microenvironment driven by dying and senescent irradiated tumor and normal tissue cells and the concomitant cascade of danger signals, chemokines, and cytokines which stimulate immune cell recruitment and activation. Moreover, the irradiated state of tumor cells bears interesting analogy to the anti-viral state, since fragments of nuclear and mitochondrial DNA that are released into the cytosol can stimulate cytosolic nucleic acid sensors to produce intra-tumoral type I interferons which are essential to (re-)activate the cancer immunity cycle and (re-)invigorate systemic anti-tumor T-cell responses. Apart from these tumor adjuvanticity enhancing effects, several reports have also described increased tumor antigenicity upon radiotherapy originating from radiation-induced exposure of neo-antigens. Collectively, radiotherapy thus may serve as a means of personalized in situ vaccination which can synergize with immune checkpoint inhibition and may help to undermine primary resistance. First clinical experiences have shown that scheduling and dosing of such combined modality treatment regimens are challenging. Moreover, recent preclinical evidence suggests that particularly the role of radiation-induced cytokines and interferons appears to be complex in such combined modality settings due to their ambiguous effects on tumor and immune cells in the tumor microenvironment. The signaling cascades that orchestrate immune cell (re-)activation and cell fate decisions in irradiated tumor cells, including tumor cell survival, proliferation, and/or metastasis formation, are intimately interconnected and require further in-depth investigation.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Immune checkpoint inhibition
- Immunotherapy
- Radiotherapy
- Head and neck squamous cell carcinoma
- Abscopal effect
- In situ vaccination
Introduction
In 1895, Wilhelm Konrad Roentgen described a novel radiation quality which he termed “X-rays” [1]. The importance of this discovery was immediately recognized by the scientific community and was spread rapidly across the globe. It reached Émil Grubbé in Chicago: a 21-year old student who was attending Hahnemann Medical School at that time. He was probably the first who used the novel radiation quality in a therapeutic setting in order to treat cancer–not even one year after Roentgen's discovery [2, 3]. This was the beginning of radiotherapy. In 1908, the first case report on what today would be classified as an “abscopal effect” of radiotherapy was published: A case of head and neck cancer described by H.D. McCulloch who also presented his hypothesis on how “immunity” contributed to spontaneous tumor regression upon irradiation of the “lymphatic glands” [4]. Since then, radiotherapy has gone through a series of impressive technical improvements and physical refinements and has evolved to a central treatment modality for various types of solid cancers, including head and neck squamous cell carcinoma (HNSCC) [5].
Radiotherapy and Immunotherapy in HNSCC Treatment
For locally advanced HNSCC, radiotherapy is implemented in definitive or adjuvant settings. State-of-the art techniques include intensity-modulated and volume-modulated arc treatment protocols in daily fractions of 1.8–2.0 Gy, alone or combined with concomitant chemotherapy [6, 7]. In recurrent or metastatic disease stages, immune checkpoint inhibition has emerged as a central part of the standard-of-care [8], together with the EXTREME chemotherapy protocol involving 5-fluorouracil, cisplatin/carboplatin, and cetuximab [9], and/or stereotactic body radiotherapy with high single doses and steep dose gradients as a palliative option [10].
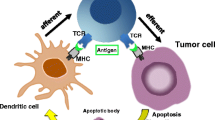
Clinical responses upon immune checkpoint inhibition are impressive but remain limited to a minority of patients [11, 12]. Primary resistance of never-responders is considered to derive from host- and tumor-specific characteristics, the latter comprising immune checkpoint activity, tumor immune contexture, tumor mutational burden, (neo-)antigen load, and others (Fig. 2.1). Secondary resistance of initially responding patients in addition, appears to be driven predominantly by irreversible T-cell exhaustion and therapy-induced selection of tumor cell clones with mutations in critical genes involved in the immune checkpoint response [13, 14].
Immunological Effects of Radiotherapy
With particular focus on primary resistance against immune checkpoint inhibition, scientific interest of preclinical and clinical researchers currently aims at the development and evaluation of combined modality treatment approaches, for instance in combination with radiotherapy: Can the immune contexture be altered in order to convert immunologically cold tumors into hot ones? And can tumor-antigenicity be increased, for instance by enforcing the presentation of neo-antigens? In this regard, the immunological implications of radiotherapy and the anecdotally reported abscopal effects are of interest. It took nearly 50 years until a term was coined for H.D. McCulloch's initial observation, and it was Robert Mole who defined it: Abscopal effects are radiation effects at a distance from the irradiated volume but within the same organism [15]. Meanwhile, this rather general definition is being nearly exclusively used in (oligo-)metastatic tumor settings describing the phenomenon of tumor regression at out-of-field locations distant from the primary site of local (radio-)therapy. In-depth preclinical analyses have shown that the underlying driving force of this phenomenon is a (re-)activation of systemic anti-tumor immune mechanisms and the cancer immunity cycle [16]. Irradiated tumor and normal tissue cells stimulate the recruitment and activation of antigen-presenting cells (APCs) which capture tumor antigen, migrate to the draining lymph nodes, and prime tumor-specific T-cell responses, particularly CD8+T-cell responses, which finally contribute to local and distant lesion regression. The basic idea of this concept and pioneering preclinical data for its validation were provided by Sandra Demaria and Silvia Formenti. From their and others’ experiments with mouse tumor models in which irradiation of the primary tumor stimulated regression of a secondary out-of-field tumor in a T-cell-dependent manner, they concluded that radiation can generate an in situ cancer vaccine [17,18,19,20,21].
An effective cancer vaccine consists of tumor-specific antigens, i.e. tumor-associated antigens or neo-antigens, and immune cell activating adjuvants. Accumulating evidence suggests that radiotherapy can affect both (Fig. 2.2).
The irradiated state of tumor cells bears interesting analogy to the anti-viral state [22]. Fragments of nuclear and mitochondrial DNA that are released into the cytosol can stimulate cytosolic nucleic acid sensors to mount an intra-tumoral type I interferon response which is essential to (re-)activate the cancer immunity cycle and (re-)invigorate systemic anti-tumor T-cell responses [23, 24]. The optimal irradiation dose and regimen to trigger these mechanisms are still under debate and seem to reveal non-linear dose–response behavior. Both, super-hypofractionated protocols (i.e. 3 × 8 Gy) as well as low dose radiotherapy with single fractions of around 2 Gy were reported to be effective in this regard [25,26,27].
Apart from the intra-tumoral type I interferon response which essentially contributes to the maturation and activation of APCs in the irradiated lesion, cytosolic DNA fragments and persisting DNA damage also determine the overall cell fate in response to radiotherapy. This strongly shapes the adjuvanticity of irradiated cells as well. Whereas non-malignant cells with functional cell cycle checkpoints commonly undergo cellular senescence upon irradiation, tumor cells often fail to properly arrest in cell cycle until the damage is repaired. In consequence, they experience several rounds of aberrant mitosis and finally commit to cell death of different morphotypes [28,29,30]. Depending on a spectrum of physical and biological parameters, including radiation quality and dose, origin and genetic repertoire of the irradiated cells, and the functionality of cell cycle checkpoints, regulated forms of apoptotic or necrotic morphology can be observed. In tumors of epithelial origin, such as HNSCC, the regulatory machinery of apoptotic cell death is frequently perturbed, and different forms of regulated necrosis appear to be dominating in response to irradiation, including but not limited to necroptosis, ferroptosis, pyroptosis, and parthanatos [31, 32] which–although regulated via different signaling cascades–all share in common that the plasma membrane disintegrates and cellular contents are released [30, 33]. Danger signals and/or damage-associated molecular patterns (DAMPs) leaking out of the dying cells activate pattern recognition receptors on neighboring cells, endothelial cells, and immune cells, and trigger an immunological reprogramming of the tumor microenvironment [29, 30].
If cell cycle checkpoint function is operational, tumor cells can commit to irradiation-induced cellular senescence. Similar to irradiated non-malignant cells, they arrest in cell cycle, increase in size, and reshape their intercellular connections and stress fibers. They produce a wide spectrum of cytokines, chemokines, and growth factors, the so-called senescence-associated secretory phenotype or SASP, which exerts multiple effects in the tumor microenvironment [34, 35]. SASP factors can contribute to vascular remodeling and immune cell recruitment [26, 36]. On the contrary, they can also support cancer cell stemness, therapy resistance, tumor repopulation, and invasion [37, 38]. So, radiotherapy-induced senescence and the corresponding secretome present as double-edged swords which can precondition the tumor microenvironment for immune checkpoint inhibition and at the same time can drive (radio-)therapy resistance and tumor progression. Accordingly, the current discussion about the implementation of broad-range senolytic and/or senomorphic drugs in the context of multi-modal cancer therapy should also include selective targeting of distinct SASP cytokines [39, 40].
Apart from elevated tumor adjuvanticity, several reports have described increased tumor antigenicity upon radiotherapy originating from radiation-induced expansion of the major histocompatibility class I/II ligandome and the exposure of neo-antigens [41,42,43,44]. Collectively, radiotherapy thus may serve as a means of personalized in situ cancer vaccination which can synergize with immune checkpoint inhibition and may help to undermine primary resistance against immune checkpoint inhibition.
Clinical Experiences with a Combination of Radiotherapy and Immune Checkpoint Inhibition in HNSCC
Given that the mechanisms described above are operational, the (re-)activation of systemic anti-tumor immunity by a combination of radiotherapy and immune checkpoint inhibition in preclinical model systems can be reportedly achieved on a reliable and regular basis [21]. However, clinical experiences are different. Here, the description of abscopal tumor regression remains limited to scattered case reports and few retrospective analyses. Nevertheless, the first cases were described at the very beginning of the therapeutic application of ionizing irradiation, and their numbers appear to be increasing–particularly since the advent of immune checkpoint inhibition [4, 45]. The most prominent case of abscopal lesion regression upon radiotherapy with ongoing immune checkpoint inhibition was reported by Michael A. Postow and colleagues. It was a case of metastatic melanoma, in which upon progression during anti-CTLA4 treatment stereotactic irradiation at 3×9.5 Gy was applied to a paraspinal lesion. The irradiated lesion showed a good response, and interestingly also the non-irradiated splenic lesions did regress [46]. Similar case reports can be found predominantly for melanoma, lymphoma, and lung cancer [21]. However, corroborating these case reports by higher level evidence in a randomized phase II trial has failed so far–at least for HNSCC. Sean McBride and colleagues compared inhibition of programmed cell death protein 1 (PD-1) versus PD-1 inhibition plus concomitant stereotactic body radiotherapy in unselected patients with metastatic HNSCC, and the rate of abscopal effects was the primary endpoint (i.e. objective response rate of non-irradiated lesions) (NCT02684253). No evidence of abscopal effects and no improvement in response rates were observed [47].
Encouraged by the success of palliative immune checkpoint inhibition in relapsed and/or metastatic HNSCC, its concomitant addition to curative-intent radiochemotherapy for locally advanced HNSCC is currently being investigated [48]. Despite good tolerability, efficacy data reported so far are rather disappointing. As such, JAVELIN Head and Neck 100, the first randomized, placebo-controlled phase III trial adding concomitant PD-L1 inhibition to definitive radiochemotherapy in locally advanced HNSCC led by Nancy Lee did not meet its primary objective of prolonging progression-free survival [49]. Further randomized phase II and III trials evaluating the concomitant addition of PD-1/PD-L1 blockade to radiochemotherapy and/or radiobiotherapy (e.g. KEYNOTE-412 (NCT03040999) or GORTEC 2017–01 “REACH” (NCT02999087)) are still ongoing. Yet, reported interim analyses prognosticate that at least the latter may not change the current standard-of-care for locally advanced HNSCC.
Considering the successful implementation of adjuvant immune checkpoint inhibition in other cancer entities–yet with clearly different treatment schedules–these results are rather disappointing. In the randomized phase III PACIFIC trial (NCT02125461), Scott J. Antonia and colleagues compared inhibition of programmed cell death ligand 1 (anti-PD-L1) as maintenance therapy after radiochemotherapy versus placebo in patients with stage III non-resectable non-small cell lung cancer. For both co-primary endpoints of progression-free and overall survival, the immune checkpoint inhibition arm was clearly superior [50]. Similarly, adjuvant PD-1 inhibition was also successful in patients with esophageal or gastroesophageal junction cancer as reported in CheckMate 577 (NCT02743494), a randomized, placebo-controlled phase III trial led by Ronan J. Kelly [51].
The reasons underlying these discrepant trial results need to be investigated in order to refine and optimize treatment concepts and to develop radiochemoimmunotherapy protocols with improved outcomes for patients with locally advanced HNSCC. Obviously, different strategies of immune checkpoint inhibition (anti-PD-1 or anti-PD-L1 blockade) and different immunoglobulin G (IgG) classes with different epitopes were used. These reported disparities have an impact on efficacy and safety profiles [52]. Of note, JAVELIN Head and Neck 100, KEYNOTE-412, and GORTEC 2017–01 “REACH” all rely on targeting the ligand PD-L1 and not the receptor PD-1. This may have implications for tumor-cell-intrinsic, retrograde signaling of PD-L1 which has recently been reported to support tumor cell growth, stemness, as well as DNA damage repair, and thus may drive resistance against the concomitantly administered radiochemotherapy [53, 54]. Furthermore, the treatment sequences need consideration. Immune checkpoint inhibition with concomitant radiochemotherapy provided negative results in JAVELIN Head and Neck 100, whereas adjuvant immune checkpoint inhibition after completion of radiochemotherapy was used in the successful PACIFIC and CheckMate 577 trials. Preclinical studies in diverse cancer models had shown that simultaneous immune checkpoint inhibition (with or without a loading dose prior to the start of radiotherapy) was superior to the adjuvant treatment sequence [55], and thus guided the trial designs of JAVELIN Head and Neck 100, KEYNOTE-412, and GORTEC 2017–01 “REACH”. In this regard, the lymphotoxic effects of concomitant radiochemotherapy during immune checkpoint inhibition may need to be considered. Chemoradiation of the circulating blood pool as well as of the tumor draining lymph nodes may interfere with the successful release of immune checkpoints and may be of minor importance in the context of adjuvant or neoadjuvant immune checkpoint inhibition [56]. Accordingly, the question arises if sparing of the lymph nodes–at least in the early phase of immune checkpoint inhibition–or alternative treatment sequences could be beneficial. Combinations of radiochemotherapy with adjuvant or neoadjuvant immune checkpoint inhibition for locally advanced HNSCC are currently underway [48]. Another relevant parameter is the fractionation regimen of radiotherapy. As described above, preclinical data suggest that the synergism between radiotherapy and immunotherapy reveals a non-linear dose–response relationship, and the optimal fractionation protocol for the (re-)activation of systemic anti-tumor immune mechanisms is still under debate. Presumably, there is no “one-fits-all” regimen, and entity-specific characteristics may need to be considered [57]. Along these lines, unique and so far disregarded aspects of HNSCC biology and/or immunology may render the implementation of immune checkpoint inhibition into the standard-of-care with curative intent for patients with locally advanced HNSCC so difficult.
Conclusions
-
Immune checkpoint inhibition has emerged as an integral part of the standard-of-care for recurrent and/or metastatic HNSCC, but response rates remain limited to a minority of patients.
-
Mechanisms of primary and secondary resistance comprise tumor- and host-derived factors, including immune checkpoint activity, immune contexture, tumor mutational burden, neo-antigen load, and others.
-
Preclinical studies and clinical case reports have shown that radiotherapy can function as a means of in situ cancer vaccination to (re-)activate systemic anti-tumor immunity, to synergize with immune checkpoint inhibition, and to break primary resistance against immune checkpoint inhibition.
-
Evaluation in randomized clinical trials has provided heterogeneous results, particularly for HNSCC.
-
Scheduling and dosing of combined modality treatment regimens appear to be challenging.
-
Unique aspects of HNSCC biology and/or immunology may be responsible that the combination of radiotherapy and immune checkpoint inhibition is so difficult.
References
Rontgen WC. On a New Kind of Rays. Science. 1896;3(59):227–231.
Pioneer in X-Ray Therapy. Science. 1957;125(3236):18−19.
Grubbe EH. X-ray treatment; its introduction to medicine. J Am Inst Homeopat. 1946;39(12):419–422.
McCulloch HD. On the analogy between spontaneous recoveries from cancer and the specific immunity induced by X ray irradiations of the lymphatic glands involved. BMJ. 1908;2(2494):1146–1148.
Orth M, Lauber K, Niyazi M, Friedl AA, Li M, Maihofer C, et al. Current concepts in clinical radiation oncology. Radiat Environ Biophys. 2014;53(1):1-29.
Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952.
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28.
Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928.
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127.
Siddiqui F, Patel M, Khan M, McLean S, Dragovic J, Jin JY, et al. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2009;74(4):1047–1053.
Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–3845.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867.
Perez-Ruiz E, Melero I, Kopecka J, Sarmento-Ribeiro AB, Garcia-Aranda M, De Las RJ. Cancer immunotherapy resistance based on immune checkpoints inhibitors: targets, biomarkers, and remedies. Drug Resist Updat. 2020;53: 100718.
Kluger HM, Tawbi HA, Ascierto ML, Bowden M, Callahan MK, Cha E, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J ImmunoTherapy Cancer. 2020; 8(1).
Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26(305):234–241.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153.
Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496.
Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695.
Brix N, Tiefenthaller A, Anders H, Belka C, Lauber K. Abscopal, immunological effects of radiotherapy: narrowing the gap between clinical and preclinical experiences. Immunol Rev. 2017;280(1):249–279.
McGee HM, Marciscano AE, Campbell AM, Monjazeb AM, Kaech SM, Teijaro JR. Parallels between the antiviral state and the irradiated state. J Natl Cancer Inst. 2021;113(8):969–979.
Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470.
Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852.
Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602.
Walle T, Kraske JA, Liao B, Lenoir B, Timke C, von Bohlen Und Halbach E, et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8. Sci Adv. 2022;8(12):eabh4050.
Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. 2017;67(1):65–85.
Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500.
Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21(2):120–134.
Lauber K, Ernst A, Orth M, Herrmann M, Belka C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol. 2012;2:116.
Yang Y, Wu M, Cao D, Yang C, Jin J, Wu L, et al. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci Adv. 2021;7(41):eabf6290.
Adjemian S, Oltean T, Martens S, Wiernicki B, Goossens V, Vanden Berghe T, et al. Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis. 2020;11(11):1003.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541.
Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118.
Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94.
Ruscetti M, Morris JPt, Mezzadra R, Russell J, Leibold J, Romesser PB, et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell. 2020;181(2):424–41 e21.
Schoetz U, Klein D, Hess J, Shnayien S, Spoerl S, Orth M, et al. Early senescence and production of senescence-associated cytokines are major determinants of radioresistance in head-and-neck squamous cell carcinoma. Cell Death Dis. 2021;12(12):1162.
Nicolas AM, Pesic M, Engel E, Ziegler PK, Diefenhardt M, Kennel KB, et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell. 2022; 40(2):168–84 e13.
Prasanna PG, Citrin DE, Hildesheim J, Ahmed MM, Venkatachalam S, Riscuta G, et al. Therapy-induced senescence: opportunities to improve anticancer therapy. J Natl Cancer Inst. 2021;113(10):1285–1298.
Wang L, Lankhorst L, Bernards R. Exploiting senescence for the treatment of cancer. Nat Rev Cancer. 2022;22(6):340–355.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271.
Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–1851.
Lussier DM, Alspach E, Ward JP, Miceli AP, Runci D, White JM, et al. Radiation-induced neoantigens broaden the immunotherapeutic window of cancers with low mutational loads. Proc Natl Acad Sci U S A. 2021; 118(24).
Lhuillier C, Rudqvist NP, Yamazaki T, Zhang T, Charpentier M, Galluzzi L, et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest. 2021;131(5).
Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–510.
Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931.
McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39(1):30–37.
Nenclares P, Rullan A, Tam K, Dunn LA, St John M, Harrington KJ. Introducing checkpoint inhibitors into the curative setting of head and neck cancers: lessons learned, future considerations. Am Soc Clin Oncol Educ Book. 2022;42:1–16.
Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450–462.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350.
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203.
Banna GL, Cantale O, Bersanelli M, Del Re M, Friedlaender A, Cortellini A, et al. Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm. Oncol Rev. 2020;14(2):490.
Kornepati AVR, Vadlamudi RK, Curiel TJ. Programmed death ligand 1 signals in cancer cells. Nat Rev Cancer. 2022;22(3):174–189.
Kornepati AVR, Boyd JT, Murray CE, Saifetiarova J, de la Pena AB, Rogers CM, et al. Tumor intrinsic PD-L1 promotes DNA repair in distinct cancers and suppresses PARP inhibitor-induced synthetic lethality. Cancer Res. 2022;82(11):2156–2170.
Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23(18):5514–5526.
Buchwald ZS, Nasti TH, Lee J, Eberhardt CS, Wieland A, Im SJ, et al. Tumor-draining lymph node is important for a robust abscopal effect stimulated by radiotherapy. J ImmunoTherapy Cancer. 2020;8(2).
Demaria S, Guha C, Schoenfeld J, Morris Z, Monjazeb A, Sikora A, et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose?. J ImmunoTherapy Cancer. 2021;9(4).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Brix, N., Lauber, K. (2023). Immune Checkpoint Inhibition and Radiotherapy in Head and Neck Squamous Cell Carcinoma: Synergisms and Resistance Mechanisms. In: Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, JP., Nicolai, P., O'Sullivan, B. (eds) Critical Issues in Head and Neck Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-23175-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-23175-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23174-2
Online ISBN: 978-3-031-23175-9
eBook Packages: MedicineMedicine (R0)