Abstract
During the past few years, we have been witnesses of a critical juncture in the history of cancer therapy; indeed, immunotherapy has been introduced initially in melanoma trials and has been gradually incorporated in the treatment algorithm of a variety of malignancies in multiple settings. Immune checkpoint inhibitors (ICIs), the most widely used immunotherapy drugs, are monoclonal antibodies that target specific immune checkpoints such as Programmed Cell Death-1 (PD-1) and Cytotoxic T-lymphocyte-Associated protein 4 (CTLA-4). Response to ICIs is characterized by marked durability, but despite a great enthusiasm that accompanied the results of phase III clinical trials, a large proportion of patients do not derive benefit from ICIs. In addition, treatment with ICIs may be associated with several atypical patterns of response, such as pseudoprogression and hyperprogression. In this chapter, we aim to illustrate current data on patterns of response to immunotherapy with focus on head and neck cancer.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
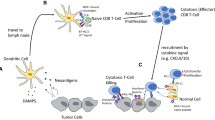
According to the cancer immunoediting theory, the three “Es” of cancer immunoediting capture the immune system’s role in protecting against tumor development. Initially and during the process of cancer immunosurveillance, the immune system successfully eliminates cancer cells through effective neoantigen processing and activation of effector T cells [1, 2]. Subsequently, the tumor evades the immune system and equilibrium is attainable when the tumor is dormant but not eradicated. Eventually and through genetic instability that fosters the outgrowth of immunosuppressive cells that disrupt the immune system, cancer eludes the immune system and avoids elimination by effector T cells. During the escape phase, cancer progresses and becomes clinically evident.
Tumors use complex, overlapping mechanisms to evade the immune system, such as inhibition of tumor antigen presentation, secretion of immunosuppressive factors and recruitment of immunosuppressive cells [3, 4]. In addition, cancer cells can dysregulate checkpoints (inhibitory) and activating signals that are responsible for the orchestration of immune response [5]. Targeting inhibitory immune checkpoints has emerged as an evolving strategy of active immunotherapy, aimed at promoting a sustainable immune response. The most studied and clinically relevant immune checkpoint pathway is the Programmed Cell Death-1 (PD-1)/Programmed Cell Death Ligand-1 (PD-L1) pathway. PD-1 is a T-cell co-stimulatory receptor commonly expressed on activated T cells, B cells and monocytes, whereas PD-L1 is expressed on tumor cells and several immune cells, such as activated T cells, B cells and Natural Killer (NK) cells [6]. Binding of PD-1 to its ligands PD-L1/PD-L2 leads to effector T cell exhaustion, repression of anti-tumor response, suppression of tumor immunity and subsequent tumor outgrowth. Anti-PD1-1 antibodies such as pembrolizumab and nivolumab successfully block PD-1/PD-L1/L2 interaction, triggering antitumor response through activation of effector T cells.
During the last 7 years, immune checkpoint inhibitors (ICIs) have been incorporated in everyday clinical practice and are now approved for a multitude of indications in numerous solid tumors, including squamous cell carcinoma of the head and neck (HNSCC). Despite great enthusiasm originating from the barrage of information and favorable results of clinical trials, it has become increasingly challenging to optimally assess the clinical benefit associated with ICIs. In addition, the use of these immunotherapeutic agents has brought to the forefront atypical patterns of response, which are distinct from those encountered with chemotherapy or targeted therapies.
In this chapter, we seek to illustrate available data on different types of responses to ICIs focusing on HNSCC.
Immunotherapy in Head and Neck Cancer
Cancer cells have the ability to generate an immunosuppressive tumor microenvironment (TME) to promote immune escape and tumor evolution [7]. In HNSCC, TME has an inflamed phenotype, characterized by an abundance of immune cells such as cytotoxic T cells (CTLs), immunosuppressive cells such as T regulatory cells (Tregs) and Myeloid-Derived Suppressor Cells (MDSCs) and production of Interferon gamma (ΙFN-γ) [8]. This phenotype is suggestive of a pre-existing anti-tumor immune response that was suddenly inhibited by the creation of an immunosuppressive TME by the tumor cells [9]. Thus, HNSCC represents a disease characterized by profound immunogenicity favoring a clinical response to immunotherapy.
Anti-PD-1 antibodies nivolumab and pembrolizumab have been approved in the platinum refractory recurrent/metastatic (R/M) setting since 2016. Checkmate 141 was a landmark, randomized phase III study that evaluated the clinical efficacy of nivolumab versus standard of care (physician’s choice of either weekly docetaxel, methotrexate or cetuximab) in platinum refractory disease [10]. This study was the first to show an overall survival (OS) benefit in favor of an ICI (median OS 7.1 for nivolumab vs. 5.5 months for investigator’s choice therapy, HR 0.70; 97.73% CI, 0.51–0.96, p = 0.01) in addition to a more favorable toxicity profile (13.1% of grade 3–4 events in the nivolumab arm vs. 35.1%) [10]. The benefit of nivolumab was prominent regardless of crossover that was allowed in the trial after protocol amendment and irrespectively of PD-L1 status [Tumor Positive Score (TPS)≤ 1% vs. >1%]. Notably, a trend for improved outcomes was demonstrated for PD-L1 positive and human papillomavirus (HPV)-associated disease; however, PD-L1 expression and p16 status were not required for enrollment and were therefore unknown for a large proportion of patients.
Pembrolizumab was granted accelerated approval for platinum-refractory R/M HNSCC based on the findings of the phase IB Keynote 012 clinical trial that showed an overall response rate of 18% and durable responses that lasted more than 6 months in 85% of responders. OS at 12 months was 38% in the updated follow-up [11]. In the confirmatory phase III Keynote 040 trial, pembrolizumab induced a clinically meaningful prolongation of OS versus standard of care (SOC; methotrexate, 3-weekly docetaxel or cetuximab); (8.4 months with pembrolizumab vs. 6.9 months with SOC [hazard ratio 0. 80, p = 0. 0161]) [12]. Increased efficacy of pembrolizumab was observed in patients with either PD-L1 combined positive score (CPS) ≥1 or TPS ≥50% compared to their counterparts in a post-hoc exploratory analysis. Thus, European Medicine’s Agency (EMA) approved pembrolizumab only for patients whose tumors express PD-L1 TPS ≥50%.
The phase III Keynote 048 clinical trial has resulted in the incorporation of pembrolizumab in the treatment algorithm of R/M HNSCC in the first-line setting. In this study, 882 patients with platinum-sensitive disease were randomized to either pembrolizumab monotherapy, pembrolizumab plus chemotherapy or the SOC (EXTREME regimen, platinum/fluorouracil plus cetuximab). PD-L1 CPS score, defined as the ratio of PD-L1-positive cells (tumor cells, lymphocytes, and macrophages) to the total number of tumor cells ×100 was used as a stratification factor. The study was not powered to detect differences in efficacy between the two immunotherapy arms that were both compared to SOC. Results were impressive, showing a significant prolongation of OS with pembrolizumab monotherapy in the PD-L1 CPS ≥20 (14.9 months vs 10.7 months for EXTREME) and CPS ≥1 groups (12.3 months vs 10.3 months) and non-inferior OS in the intention to treat (ITT) population (11.6 months in the pembrolizumab group vs 10.7 months in the EXTREME group, respectively). In addition, pembrolizumab in combination with chemotherapy demonstrated OS benefit vs the EXTREME regimen in the PD-L1 CPS ≥20 (14.7 months vs 11.0 months), PD-L1 CPS ≥1 (13.6 months vs 10.4 months), and ITT populations (13.0 months vs 10.7 months) [13]. In a post-hoc analysis that was recently published, in the CPS <1 subgroup, neither pembrolizumab nor pembrolizumab plus chemotherapy showed benefit in OS versus the EXTREME regimen, but the analysis was inconclusive due to the small number of patients in this population [14]. For the CPS 1–19 subgroup, pembrolizumab plus chemotherapy significantly improved OS compared to EXTREME (HR = 0.71, p = 0.01), whereas prolongation of OS with pembrolizumab monotherapy was non-significant versus EXTREME (HR = 0.86, p = 0.25) [14].
Combinations of anti-CTLA4 and anti-PD1/PD-L1 inhibitors have failed to show survival benefit compared to EXTREME in the first-line R/M setting. In the recent Checkmate 651 study that assessed the clinical efficacy of the combination of nivolumab plus the anti-CTLA-4 antibody ipilimumab versus the EXTREME regimen in the first-line setting of R/M HNSCC, no statistically significant increase of OS was observed either in the total population or the CPS ≥20 population [15]. However, 2-year rates of OS were very encouraging for patients receiving nivolumab and ipilimumab (41% and 34% in the CPS ≥20 and CPS ≥1 groups, respectively). Similarly, the phase III KESTREL study, a global phase III study randomizing patients with previously untreated R/M HNSCC to treatment with the anti-PD-L1 antibody durvalumab monotherapy versus the combination of durvalumab plus the anti-CTL4 antibody tremelimumab versus the SOC EXTREME regimen [16]. KESTREL failed to reach the primary endpoint of OS in patients with PD-L1 TPS ≥50% or tumor-infiltrating immune cells (IC) ≥25% according to a press release from AstraZeneca in February 2021.
In the second-line setting, Checkmate 714 [17], a—randomized phase II study comparing nivolumab plus ipilimumab to nivolumab plus placebo failed to meet its primary endpoints of ORR and duration of response in platinum-refractory patients according to a press release from Bristol-Myers Squibb in January 2020. Moreover, the phase III EAGLE trial [18], designed to test the clinical activity of durvalumab or durvalumab plus tremelimumab versus SOC therapy (cetuximab, methotrexate, a taxane, or a fluoropyrimidine) in platinum-refractory disease, did not show any superiority of either durvalumab monotherapy or thecombination with tremelimumab versus conventional chemotherapy.
In conclusion, ICIs such as nivolumab and pembrolizumab have shown remarkable efficacy in HNSCC and their use has been incorporated in the treatment algorithm in both first- and second-line settings, yielding improved clinical outcome both in terms of efficacy and toxicity.
Patterns of Response to Immune Checkpoint Inhibitors
Immunotherapy has revolutionized the field of oncology and has changed the way physicians evaluate the clinical benefit of therapy and treatment sequelae. Compared to chemotherapy, ICIs display pharmacokinetic and pharmacodynamic differences. For example, for the majority of chemotherapy drugs, the biological effect increases as the plasma concentration of the drug rises and the intensity of adverse events is dose-dependent [19]. In the context of chemotherapy efficacy, the term therapeutic window refers to a range of doses which optimize between efficacy and toxicity, reaching the greatest therapeutic benefit without causing unacceptable adverse events. On the contrary, efficacy of immunotherapy is not characterized by a dose-dependent treatment effect, but rather a more delayed effect with a variable proportion of long-term survivors (plateau of the curve) [20]. In addition, immune-related adverse events (irAEs) can have a delayed onset and prolonged duration compared to chemotherapy side effects [19].
Most importantly, the use of ICIs has been linked to the development of several atypical patterns of treatment response. For example, ICIs can cause tumor shrinkage that is maintained over time despite treatment discontinuation (prolonged response), minimal or non-existent modifications in target lesions (stable disease), initial tumor progression followed by shrinkage on subsequent imaging (pseudoprogression) or unexpectedly rapid tumor expansion with clinical patient deterioration (hyperprogression) [21].
Pseudoprogression
The phenomenon of pseudoprogression was first described in a phase II trial evaluating the efficacy and toxicity of ipilimumab in 155 patients with previously treated advanced melanoma. Di Giacomo et al. described the case of one patient that experienced an initial increase in tumor size of lung metastases followed by remarkable tumor regression for a prolonged duration [22]. Pseudoprogression has been since then defined as a temporary increase of tumor size or burden followed by a subsequent tumor reduction on following imaging.
Pseudoprogression does not represent actual tumor growth. It has been hypothesized that it biologically originates from the infiltration of the tumor by activated T cells that are prompted to the tumor site following treatment with ICIs, edema and necrosis [23]. Indeed, in the original report by Di Giacomo et al., a biopsy performed at the time of initial progression showed infiltration by CTLs and granzyme B+ indicating a functional T cell population [22].
Using classical radiographic criteria for assessment of response to immunotherapy, pseudoprogression might lead to early discontinuation of treatment in clinical trials in patients that may have had a later response to immunotherapy. The development of immune-specific related response criteria (irRC) that were introduced based on data from patients treated with ipilimumab in melanoma trials, mirrors this exact need of being able to continue immunotherapy in patients with atypical patterns of response, such as pseudoprogression or mixed response, without the false alarm of disease progression (PD) [24]. These criteria differed from classical RECIST in the definition of partial response (immune-related partial response, irPR; decrease in ≥50% in disease burden), and immune-related PD (irPD; an increase in tumor burden by ≥25% relative to nadir).
More recently, immune-related RECIST criteria (irRECIST) have been established as a result of a further refinement of radiologic criteria to better evaluate responses to immunotherapy. These criteria were formulated as a mixture of iRC and RECIST criteria and the main differences compared to iRC are (a) the use of unidimensional measurements; while in iRC criteria lesions are measured in two dimensions (longest diameter and longest perpendicular diameter), in irRECIST, lesions are measured using only the longest diameter, (b) irPR is defined as ≥30% from baseline and (c) irPD must be confirmed with a second imaging study at least 4 weeks later to enable development of delayed immune response. Thus, irPD is confirmed only if the second scan demonstrates new findings or new unequivocal progression compared to the first one. In irRECIST, repeat assessment is allowed up to 12 weeks [23, 25]. Last, iRECIST criteria were developed in March 2017 following an expert consensus. These modified criteria introduce new nomenclature, such as an immune unconfirmed progressive disease (iUPD) or immune confirmed PD (iCPD). More specifically, when PD based on RECIST 1.1 criteria is observed, this is termed iUPD and it needs confirmation with subsequent imaging to be regarded as true progression (iCPD), which is defined as an increase of at least 5 mm of the total measurements of target lesions from iUPD. Criteria iRECIST suggest subsequent imaging to be performed between 4 and 8 weeks post iUPD, compared to 4–12 weeks in irRECIST [26].
In head and neck cancer, the incidence of pseudoprogression has been reported as below 3% in several studies. Of note, responses were assessed by RECIST criteria in the majority of the studies. Seiwert et al. reported the results of the PD-L1 positive cohort of the phase Ib Keynote 012 study, in which patients with R/M HNSCC received pembrolizumab as second- or later-line of treatment [27]. In this study, among 45 patients assessed by central review for response, one patient experienced an atypical response before a confirmed complete response, probably a pseudoprogression, although it is not clear whether the increase in tumor size was observed clinically or on imaging. In the expansion cohort of the same study, no pseudoprogression was noted [28]. In Keynote 040, responses to pembrolizumab were also assessed based on RECIST criteria [12]. As shown in the swimmer’s plot in the appendix of the Keynote 040 publication, nine patients were continued on pembrolizumab beyond progression and 2 of them were still on treatment 8 weeks post unconfirmed progression (based on iRECIST), indicating a 0.8% (2 out of 247 patients that received pembrolizumab) of pseudoprogression. On the other hand, Haddad et al. reported the results of treatment beyond progression with nivolumab in patients with R/M-HNSCC who participated in the Checkmate 141 trial [29]. Among 146 patients with disease progression, 62 patients continued treatment with nivolumab; in this study, continuation of immunotherapy was allowed if the drug had clinical benefit as assessed by the investigator. Among 62 patients, 15 experienced a decrease in target lesions and 3 had a confirmed PR, indicating a 1.3% incidence of pseudoprogression [29]. Last, in a retrospective report by Sridharan et al. aiming to evaluate predictors of response to immunotherapy in 100 patients with R/M-HNSCC, the rate of pseudoprogression was 1% (1 out of 100 patients) [30].
Based on iRECIST and given the rarity of pseudoprogression across tumor types (<10%) [25, 29, 31, 32], it is critical to appropriately select patients who are candidates to continue immunotherapy beyond progression by striking a balance between premature cessation and delay of a subsequent potentially efficacious treatment strategy. Key points include (a) the identification of early signs of clinical deterioration that imply a true disease progression; indeed, although there may appear to be tumor growth on initial imaging and physical examination, pseudoprogression should not be accompanied by clinical deterioration and (b) a biopsy at the time of progression that shows infiltration by immune cells might be helpful and (c) the patient should not have experienced severe adverse events. Further studies are warranted to identify potential biomarkers for immune response.
Hyperprogressive Disease (HPD)
A meticulous study of survival curves in several immunotherapy clinical trials reveals an early crossing of the curves during the first 6 months of treatment that indicates a subgroup of patients with deleterious effects induced by immunotherapy [10, 13, 33, 34]. For example, in Keynote 048, the survival curves clearly favor the EXTREME arm compared to pembrolizumab monotherapy arm during the first few months [13]. This phenomenon might be partly explained by the concept of hyperprogression, which represents immunotherapy-induced rapid acceleration of tumor growth kinetics [23].
HPD was first reported by Champiat et al., who conducted a retrospective study that included 218 patients with different solid tumors treated with anti-PD-1/PD-L1 inhibitors in phase I studies [35]. The authors compared the tumor growth rate (TGR-an estimation of increase of tumor volume over time) prior the initiation and post treatment with ICIs. HPD was defined as PD by RECIST criteria combined with twofold increase of the TGR. The incidence of HPD was found to be 9% in this study, and no association with tumor burden or specific tumor type was noted. However, HPD correlated with worse outcomes [35].
Following this publication, several groups, including ours, have described their experience with HPD [36,37,38,39]. The reported incidence of HPD ranges from 4 to 29% largely depending on the tumor type and the relevant definition. In HNSCC, the incidence of HPD ranges from 6 to 29%. More specific, a second group of authors from San Diego added time to treatment failure (TTF) to the definition of HPD, which was defined as TTF <2 months, >50% increase in tumor burden compared with the size prior to treatment (notably, the first assessment of treatment response was performed within two months after immunotherapy initiation), and twofold increase in progression pace. They conducted a retrospective analysis and Next-Generation Sequencing (NGS) genomic analysis in a cohort of 155 patients with various solid tumors, among which 11 had HNSCC. One out of 11 patients with HNSCC had HPD (incidence 1%). Interestingly, this study found a correlation of MDM2/4 amplification and EGFR alterations with TTF <2 months and a correlation of MDM2 amplification with increased incidence of HPD [37].
Subsequently, Saâda-Bouzid et al. retrospectively analyzed and compared the TGR before immunotherapy and at first imaging in a cohort of 34 patients with HNSCC [38]. HPD was defined as TGR ratio ≥2. In this study, the incidence rate of HPD was found to be 29%. In addition, HPD significantly correlated with a regional recurrence, but not with local or distant recurrence and was associated with a shorter PFS (2.9 vs. 5.1 months, P = 0.02) [38].
Our group reported data regarding HPD in a cohort of 117 patients with R/M HNSCC. Tumor growth kinetics were assessed and were available in 49 patients [39]. Using the same definition as the French group [38], HPD was documented in 15.4% of the whole cohort. Occurrence of HPD was associated with worse PFS (1.8 months in patients with HPD vs. 6.1 months in patients with non-HPD, p = 0.0001) and OS (6.53 months in patients with HPD vs. 15 months in patients with non-HPD, p = 0.0018) and clinical parameters such as primary site in the oral cavity and administration of immunotherapy in the second/third-line setting. In addition, EGFR amplification was solely documented in patients with HPD, although not statistically significant [39].
Kanjanapan et al. reviewed the records of 352 patients with various solid tumors that participated in early clinical trials [40]. Among 32 patients with HNSCC that were included in the study, two had HPD (incidence 6%). No association with clinical characteristics (except female sex) or survival was found in this study. Most recently, Park et al. reported the incidence and clinical effect of HPD in 125 patients with R/M-HNSCC treated with immunotherapy in 11 Korean centers [41]. HPD was observed in 14.4% of patients, and a correlation was found with younger age, primary site in the oral cavity (similar to the findings of our group) and prior irradiation. Interestingly, no correlation with survival was reported and a clinical benefit with subsequent treatment was shown.
In a recently published systematic review and meta-analysis of 24 studies with 3109 patients, the authors sought to summarize the definitions and incidence of HPD across different tumor types [42]. A pooled incidence of HPD equal to 13.4% was found, with a wide range due to the various definitions of HPD. The authors concluded that the incidence of HPD might be miscalculated if only TG kinetics are used, because no clinical criteria are taken into consideration [42]. In addition, TG kinetics are complex to estimate and require pre-baseline CT scans, which are not easily available particularly in treatment-naïve patients where immunotherapy is being administered as first-line treatment. The timeframe for a second imaging assessment is also a point of discussion, as it should be less than two months to enable capture of hyperprogressors at an early timepoint. Nevertheless, clinical criteria to evaluate clinical deterioration, such as TTF or increase in tumor burden, might not be sufficient to accurately estimate clinical status.
Several potential mechanisms have been implicated in the development of HPD. First, blockade of the PD-1/PD-L1 immune checkpoint might result in the recruitment of Tregs that create an immunosuppressive TME. Second, immune blockade might be counteracted by the activation of several alternative checkpoints contributing to T cell exhaustion. In addition, several immunosuppressive cells such as MDSCs and macrophages might lead to the production of cytokines and other factors, such as IL-10 and IFN-γ. Furthermore, it can be hypothesized that PD-1/PD-L1 blockade can trigger the activation of tumorigenic signaling pathways [43, 44].
Given retrospectivity of data and the lack of validated biomarkers, many researchers question the originality of HPD and claim that it could be the natural evolution of a tumor unresponsive to immunotherapy [21]. Although scarce genomic and clinical associations have been reported, these might be random findings. In addition, hyperprogression has been less frequently reported in patients receiving chemotherapy and targeted therapy and might not be uniquely observed in immunotherapy-treated patients. Randomized trials comparing immunotherapy with no treatment, albeit ethically rather unacceptable, would help elucidate the phenomenon of HPD. This could be possibly feasible at later lines of therapy, where no standard treatment exists; for example, nivolumab has been compared to placebo in the third line setting of gastric/gastroesophageal junction cancer [45]. Nevertheless, patients do experience rapid tumor growth following immunotherapy, and we must try to exploit clinical experience and molecular characterization originating from expert centers.
Conclusions
In conclusion, it becomes increasingly clear that patterns of response and progression to immunotherapy differ from those observed with chemotherapy and targeted agents. Although it has been rarely reported in head and neck cancer, pseudoprogression is a well-described phenomenon that has led to the development of immune-specific related response criteria that enable continuation of treatment beyond progression. On the other hand, HPD on anti-PD1 agents has been reported in 9–29% of head and neck cancer patients. The heterogeneity of HPD definitions and the retrospectivity of reported data have created a controversy between real hyperprogression and natural history of disease progression. To this direction, there is an urgent need to identify biomarkers for prediction of progression or hyperprogression with the view to optimize treatment outcomes.
References
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71.
Hsieh RW, Borson S, Tsagianni A, Zandberg DP. Immunotherapy in recurrent/metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2021;11: 705614.
Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Economopoulou P, Kotsantis I, Psyrri A. Tumor Microenvironment and Immunotherapy Response in Head and Neck Cancer. Cancers. 2020;12(11).
Cheng G, Dong H, Yang C, Liu Y, Wu Y, Zhu L, et al. A review on the advances and challenges of immunotherapy for head and neck cancer. Cancer Cell Int. 2021;21(1):406.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67.
Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119(2):153–9.
Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–67.
Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–28
Burtness B, Rischin D, Greil R, Soulieres D, Tahara M, de Castro G, Jr, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol: Off J Am Soc Clin Oncol. 2022:JCO2102198.
Argiris A, Harrington K, Tahara M, Ferris RL, Gillison M, Fayette J, et al. LBA36 Nivolumab (N) + ipilimumab (I) vs EXTREME as first-line (1L) treatment (tx) for recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): Final results of CheckMate 651. Ann Oncol. 2021;32:S1310–1.
Seiwert TY, Weiss J, Baxi SS, Ahn M-J, Fayette J, Gillison ML, et al. A phase 3, randomized, open-label study of first-line durvalumab (MEDI4736) ± tremelimumab versus standard of care (SoC; EXTREME regimen) in recurrent/metastatic (R/M) SCCHN: KESTREL. J Clin Oncol. 2016;34(15 suppl):TPS6101-TPS.
Haddad R, Gillison M, Ferris RL, Harrington K, Monga M, Baudelet C, et al. Double-blind, two-arm, phase 2 study of nivolumab (nivo) in combination with ipilimumab (ipi) versus nivo and ipi-placebo (PBO) as first-line (1L) therapy in patients (pts) with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN)—CheckMate 714. Ann Oncol. 2016;27:vi350.
Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol: Off J Eur Soc Med Oncol. 2020;31(7):942–50.
Puzanov I, Diab A, Abdallah K, Bingham C, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immuno Therapy Cancer. 2017;5.
Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801.
Adashek JJ, Subbiah IM, Matos I, Garralda E, Menta AK, Ganeshan DM, et al. Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer. 2020;6(3):181–91.
Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol, Immunother: CII. 2009;58(8):1297–306.
Borcoman E, Nandikolla A, Long G, Goel S, Tourneau CL. Patterns of Response and Progression to Immunotherapy. Am Soc Clin Oncol Educ Book. 2018;38:169–78.
Hoos A, Eggermont AMM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved Endpoints for Cancer Immunotherapy Trials. JNCI: J Natl Cancer Inst. 2010;102(18):1388–97.
Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol. 2017;44(1):3–7.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–52.
Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–65.
Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol: Off J Am Soc Clin Oncol. 2016;34(32):3838–45.
Haddad R, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Treatment beyond progression with nivolumab in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) in the phase 3 checkmate 141 study: A biomarker analysis and updated clinical outcomes. Ann Oncol. 2017;28:v372–3.
Sridharan V, Rahman RM, Huang RY, Chau NG, Lorch JH, Uppaluri R, et al. Radiologic predictors of immune checkpoint inhibitor response in advanced head and neck squamous cell carcinoma. Oral Oncol. 2018;85:29–34.
Long GV, Weber JS, Larkin J, Atkinson V, Grob J-J, Schadendorf D, et al. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017;3(11):1511–9.
George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, et al. Safety and efficacy of Nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol. 2016;2(9):1179–86.
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res: Off J Am Assoc Cancer Res. 2017;23(8):1920–8.
Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–52.
Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after Immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res: Off J Am Assoc Cancer Res. 2017;23(15):4242–50.
Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol: Off J Eur Soc Med Oncol. 2017;28(7):1605–11.
Economopoulou P, Anastasiou M, Papaxoinis G, Spathas N, Spathis A, Oikonomopoulos N, et al. Patterns of Response to Immune Checkpoint Inhibitors in Association with Genomic and Clinical Features in Patients with Head and Neck Squamous Cell Carcinoma (HNSCC). Cancers. 2021;13(2).
Kanjanapan Y, Day D, Wang L, Al-Sawaihey H, Abbas E, Namini A, et al. Hyperprogressive disease in early-phase immunotherapy trials: clinical predictors and association with immune-related toxicities. Cancer. 2019;125(8):1341–9.
Park JH, Chun SH, Lee YG, Chang H, Lee KW, Kim HR, et al. Hyperprogressive disease and its clinical impact in patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with immune-checkpoint inhibitors: Korean cancer study group HN 18–12. 2020;146(12):3359–69.
Park HJ, Kim KW, Won SE, Yoon S, Chae YK, Tirumani SH, et al. Definition, Incidence, and challenges for assessment of hyperprogressive disease during cancer treatment with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e211136-e.
Camelliti S, Le Noci V, Bianchi F, Moscheni C, Arnaboldi F, Gagliano N, et al. Mechanisms of hyperprogressive disease after immune checkpoint inhibitor therapy: what we (don’t) know. J Exp Clin Cancer Res. 2020;39(1):236.
Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria J-C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15(12):748–62.
Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Economopoulou, P., Psyrri, A. (2023). Patterns of Response to Immune Oncology Drugs: How Relevant Are They in SCCHN?. In: Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, JP., Nicolai, P., O'Sullivan, B. (eds) Critical Issues in Head and Neck Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-23175-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-23175-9_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23174-2
Online ISBN: 978-3-031-23175-9
eBook Packages: MedicineMedicine (R0)