Abstract
Gender affirming surgery should provide satisfying results in terms of aesthetic and functional outcomes, therefore, no standard procedure could fit for every subject. Penoscrotal flap technique with neoclitoral-neourethral complex confreres acceptable outcomes, is reproducible in different anatomical scenarios and not involves major abdominal surgery.
All pictures are original, property of Dr. Migliozzi F.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
23.1 Introduction
The challenging male-to-female sexual reassignment surgery requires good surgical technique and well-trained surgeons. My technique has been developed after 30 years of experience and after more than 400 patients treated. Herein are reported all preoperative, intraoperative, and postoperative steps [1,2,3,4,5].
23.2 Preoperative Procedures
At the time of their first surgical visit, patients are prompted to get perineal laser hair removal to avoid hair growth inside the neovagina [6]. One month before surgery, patients must stop oestrogen therapy due to well-known potential cardiovascular risks, whereas the antiandrogenic therapy can be continued [7, 8].
23.3 Position of the Surgeons
Since our initial experiences when we perform male-to-female surgery, we do so with two surgical teams who work in the same time and sometimes all together: with the patient in lithotomic position, two surgeons are placed on either side of the patient, and other two surgeons are positioned at the perineal side (Fig. 23.1) [9, 10].
23.4 Markings
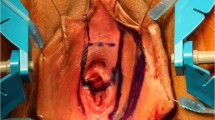
Before the beginning of the operation, we start by marking on the pubic-umbilical line, which is done for correctly locating all the structures. Another drawing is done at the scrotal level, drop shaped with the apex pointing towards the anus, which represents the scrotal flap. The correct distance between the anal verge and the apex of the drawing is 1–2 cm. The length of the scrotal flap varies between 12 and 15 cm. It is crucial that when measuring the length of the scrotal flap, there must not be tension at the apex of the skin flap after suturing. For the circumcision, another line is drawn in order to leave 1 cm of skin at the under the glans of penis.
The drawing is then continued for the length of the penis on ventral side (Figs. 23.2 and 23.3).
23.5 Devices
The sterile drape that we use for covering the patient permits to access anal-rectal area (with instruments or manually), without contaminating the operatory field. This manoeuvre is determinant in avoiding rectal injuries during blunt dissection. It consists of a water-repellent drape which can cover legs and has also a fluid collection pouch.
Power star bipolar scissors (Johnson & Johnson Medical GmbH) are very useful especially to dissect urethra.
The surgical illuminator provides cool, shadowless, deep cavity lighting. Flexible or malleable, it may be attached to most retractors or instruments, using two-side adhesives. Once attached to the instrument, its thin profile takes minimal space.
When connected to an ACMI cable and standard, 300 Watt xenon light source, it lights the cavity as if a fluorescent light was switched on inside the patient. The LightMat® brings bright, cool light where it is needed—into the surgical cavity, improving visualization, helping surgeons save time and avoid complications.
The V-LocTM wound closure device (COVIDIEN) is a new technology that eliminates the need to tie knots, so you can close incisions up to 50% faster without compromising strength and security. The V-LocTM device offers secure, fast, and effective incision closure for our patients: in particular, the absence of knots into the neovagina avoids painful dilatation manoeuvres in the postoperative period. A small (9 cm × 4 cm → 100 mL of water) or medium (12 cm × 4 cm → 130 mL of water) vaginal stent is used. It is an inflatable cylinder made of biocompatible silicone with a clamp and a connector, specifically produced for trans women.
23.6 Perineal Surgery
The surgeon positioned at the perineal side, incises the skin along the line drawn on perineum.
The first step is the bilateral orchiectomy with dissection and suturing of both the spermatic cords at the level of the external inguinal rings. The peritesticular fat is preserved as support for the labia majora. The dissection is extended through the subcutaneous tissue until reaching the corpus spongiosum, which is then isolated from the corpora cavernosa. The urethra is severed at the pubic symphysis, and the terminal portion is spatulated and longitudinally split on the ventral midline so as to obtain a Y-shape. At this point, a urethral catheter is inserted (Fig. 23.4) [9].
The position of neo-urethral meatus is ensured by applying a knot at the apex of the incision. This becomes the reference point for positioning the neoclitoris, which will be surrounded by urethral mucosa (Figs. 23.5 and 23.6). The urethral bulb is carefully removed in order to prevent its bulging during sexual arousal and pain during penetration. Running absorbable suture of the two margins of the urethra decreases the risk of postoperative bleedings.
Surgery continues with the removal of the proximal corpora cavernosa, facilitated by the placement of a traction point at the apex of tissue, paying attention to the vascular bundle.
These manoeuvres allow the exposure of the perineal tendinous centre, which is then opened while monitoring the integrity of the rectal wall through the anal access provided by the sterile drop. At this point, the surgeon creates a neo-cavity in the rectoprostatic space which will accommodate the neovagina: we usually use a LigaSure device to sever the subcutaneous tissue, while sight is aided by an aspirator provided with a light source. The knowledge of the rectoprostatic space has been acquired, thanks to a large number of pelvic postoperative MRIs administered in order to have a precise anatomical visualization of all the structures involved in the surgery. These allow for measurements of inclination, length, and distance of the neovagina and its adjacent organs [11, 12].
When the catheter’s balloon is palpated by the operator, it means that the neovagina is deep enough (Fig. 23.7). The fixation of the cul-de-sac in the rectoprostatic space is crucial to prevent the neovagina from prolapsing. Two Prolene double needle stitches are passed through the wall of the neo-cavity (anterior and posterior walls or the lateral walls), and both the ends of the sutures are passed through the penile-scrotal flap at the level of the cul-de-sac. In order to prevent prolapse, we also put another stitch through the scrotal flap and the subcutaneous tissue in proximity of the Denonvilliers’ fascia incision.
23.7 Penile Surgery
The two surgeons positioned on either side of the patient begin by performing incisions following the line previously drawn, and the penile flap is obtained by isolation from the corpora cavernosa, taking care to preserve vitality of the skin. Positioning a tourniquet around the corpora cavernosa and the urethra, it is possible to obtain a hydraulic erection using a butterfly needle and infusing saline solution into the corpora cavernosa. This is done to facilitate the blunt dissection of Buck’s fascia from the tunica albuginea. Buck’s fascia is identified and incised at a paraurethral level to avoid damage of the dorsal nerves of the penis. This procedure is done in order to permit the creation of a sensitive neoclitoris.
These structures need to be manipulated with delicate instruments, such as anatomical clamps, in order to reduce damage. For this same reason, bleeding is controlled with a bipolar coagulator.
After achieving the complete bilateral dissection of the neurovascular bundle, we insert two open tourniquets up to favourite glandulectomy. At the base of the penis, the dissection of the bundle is continued by positioning a Satinsky clamp at the level of the corpora cavernosa up to inhibit blood refilling so as to avoid wasting of blood. Removal of all corpora cavernosa avoids an eventual residual erection that can cause dyspareunia.
The glans is reduced and remodelled preserving a good amount of spongiosum tissue to create a well-vascularized neoclitoris: the dimensions of the resulting button are greater than that of the female clitoris. This is done so as to oppose eventual excessive hypotrophy of the tissues.
The bundle is folded on itself and fixed with a stitch: in this way, we create the mons pubis to better simulate the female form, and we also prevent the torsion of the flap avoiding neoclitoral ischemia. The neoclitoris is now joined to the urethral plate at the forking previously created. The suture is done in two layers, at the level of spongiosum tissue and the other at the level of urethral mucosa and neoclitoris epithelium. It is interesting to underline that the two layers have the same histological characteristics (Figs. 23.8 and 23.9) [13].
Creation of the urethra-clitoris complex is an innovative technique and presents two main advantages:
-
It allows for the neoclitoris to be in a mucosal environment.
-
The two-layer suture permits a reciprocal vascular support, useful in case of urethral or clitoral ischemia.
23.8 Tubularization of Penile-Scrotal Flap
At this point, the peritesticular fat is fixed at the perineal level. Scrotal and penile skin is sutured, using Vicryl 3/0, only bilaterally leaving an opening at the apex. We do this because no patient ever has enough skin to fully cover the entire length of the neocavity, and if we completely closed the neovaginal tube, there would be a possible loss of depth. Another option is to lengthen the penile-scrotal flap using redundant scrotal skin as a graft [14].
The two Prolene stitches we had put at prostatic and rectal level are now passed through the penile and scrotal flap. A frank demarcation between the labia minora and labia majora is obtained by placing four stitches between the outside of the penile-scrotal cylinder and the crura. Residual skin is excised. A longitudinal midline incision on the penile flap is performed in order to allow the exteriorization of the urethra-clitoris complex, catheter, and urethral meatus.
In order to avoid hypersensitivity of the neoclitoris, a cap is created from the residual preputial skin (Fig. 23.10). Now, the penile-scrotal cylinder of skin is introduced into the neo-cavity, now forming the neovagina, and the two Prolene stitches are safely knotted to hold it in place and avoid any eventual prolapse (Fig. 23.11) [15]. We have to remember the presence of ureters when we pass the two stitches into the deep part of the neocavity [16].
The surgical incision at the end of the procedure appears as a wide “U” and is closed with a V-loc running suture.
As the final step, a lubricated adjustable vaginal stent is inserted into the neovagina, making sure that it reaches the vault. The stent consists of a sealed silicone shell filled with polyurethane foam, fully adjustable by inflation (Fig. 23.12).
During insertion, the stent is deflated, only to be inflated once the position is satisfactory. After inflation, in order to avoid slipping due to the smooth surfaces of the stent, a cotton ball is always inserted in the vaginal introitus [17]. The usefulness of this vaginal stent is double: first, it prevents the vaginal walls from collapsing, and second, it is designed to permit the drainage of all secretions. A compressive packing is always done, in a criss-cross fashion, using a wide adhesive gauze. A good compression avoids postoperative bleeding and swelling and helps maintain the stent in place.
23.9 Postoperative Care
After surgery, the patient must stay in a supine position, for 2 days. During this period, it is crucial to conduct thrombo-embolic prophylaxis (heparin, antiembolic stockings, and mobilization of the feet and legs). Usually, the first day the patient is restricted to a liquid diet, and during the second day, she can begin a solid diet.
From the second day, washing of the neovagina is done by introducing a small amount of Iodine-saline solution through the stent.
On the third day, the stent is deflated and removed for the first time and disinfected with diluted sodium hypochlorite. Anti-swelling or heparin-based gels are applied on eventual haematomas.
After 4 days, the urethral catheter is removed, and the patient experiences urination for the first time. Now dilatations with rigid vaginal stents begin, and the patient is taught the correct manoeuvres for insertion and care. We usually adopt Amielle Comfort (Owen Mumford) vaginal dilators which have been designed for women experiencing vaginal discomfort and penetration problems from vaginism, dyspareunia, and gynaecological surgery.
The recommended position to insert into neovagina the lubricated dilators is to lie flat on the back with the knees bent and legs slightly apart. Alternatively, the patient can stand with one leg raised on a chair. Dilators can be washed with water and soap, and dried thoroughly ensuring all traces of soap have been removed before their insertion into the neovagina.
Psychosexual therapists teach how to do the following [18]:
-
To control breathing.
-
To relax the legs as much as possible.
-
To gently ease the lubricated dilator in an upward and backward direction as deeply as is.comfortable.
-
To leave the dilator in position for up to 5 min.
When a patient feels comfortable with using the smallest dilator, gradually move to the next size and so on. Moreover, during the first postoperative days, we suggest to use during the night the adjustable vaginal stent which allows a continuous dilation of the neovagina during the night hours.
In the first postoperative period, we suggest to repeat this procedure four to five times a day in a place that is comfortable and ensures her privacy. When no complication occurs, the patient can be discharged 5–6 days after surgery. When patients feel comfortable with inserting the larger dilators, no secretion is evident and no pain is referred, they may be ready to attempt penetrative sexual intercourse. In our experience, the timing of the first intercourse is 45–60 days after surgery.
23.10 Possible Side Effects and Complications
A frequent side effect is the development of haematoma of the labia of the neovagina which is particularly in presence of abundant skin and usually resolves spontaneously (Fig. 23.13).
If surgery lasts too much, BMI is high, and patient’s legs are not well positioned during the operation, a leg muscular contusion may develop due to the prolonged lithotomy position during operation: this rare event requires fasciotomy of the peronaeorum communis fascia to be performed as soon as possible (4–6 h after the first intervention) [19].
In our experience, an important critical step is the preparation of the neurovascular dorsal penile bundles. Injury to the arteries or nerves may result in an impaired blood supply or reduced sensation of the clitoris.
Very rare is the eventuality of total necrosis of the neoclitoris (Fig. 23.14).
Another possible complication is neo-meatal stricture or substenosis. In our opinion, it is extremely important to widely spatulate the urethra and to suture the neo-meatus with separate stitches.
Other possible major complications include the following:
-
Partial necrosis of the penile or scrotal flap.
-
Bleeding from the urethra and the neoclitoris.
-
Shortening or substenosis of the neovagina.
In case of partial or total prolapse of the neovagina, a second surgical treatment is necessary [20].
References
Belgrano E, Trombetta C, Repetto U, Pittaluga P, De Rose AF, Giuliani L. Pseudoermafroditismo maschile con persistenza delle strutture mulleriane: descrizione di un caso. Atti 58° Congr. S.I.U.-Roma, 25–28 settembre 1985. Acta Urol Ital. 1986;429–433.
Belgrano E, Repetto U, Trombetta C, Siracusano S, Bozzo W, Pittaluga P. Persistenza delle strutture mulleriane: descrizione di un caso. Rassegna Ital Chir Pediatr. 1986;28(2):120–3.
Trombetta C, Siracusano S, Gabriele M, Belgrano E. Hernia uteri inguinalis: un caso particolare di pseudoermafroditismo maschile. Atti 5° Corso Agg. Androl. di interesse chir.- Fiuggi Terme, 27–28 giugno 1988. Andrologia Chirurgica in Età Pediatrica. Acta Med Austriaca. 1989;1989:77–82.
Belgrano E, Trombetta C, Siracusano S, Desole S. Creazione di neovagina in due casi di pseudoermafroditismo. In: Atti del 63° Congr. S.I.U. (Società Italiana di Urologia)—Ancona, 9–12 Settembre 1990; 1990.
Trombetta C, Siracusano S, Desole S, Foddis G, Usai V, Belgrano E. Creazione di una neovagina in caso di pseudoermafroditismo: presentazione di due soluzioni tecniche. Atti 40° Convegno S.U.I.C.M.I.-Alghero, 23–25 Maggio 1991. Acta Urol Ital. 1992;(suppl. 5):231–234.
Belgrano E, Fabris B, Trombetta C. Il Transessualismo: identifi cazione di un percorso diag-nostico e terapeutico. Milano: E. Kurtis; 1999. p. 1–374.
Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, Kaufman JM, T’Sjoen G. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case–control study. Eur J Endocrinol. 2013;169(4):471–8. https://doi.org/10.1530/EJE-13-0493.
Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47:337–42.
Trombetta C, Buttazzi L, Liguori G, Belgrano E. Sex reassignment surgery: male-to-female transsexual operation. eUROtraining. In: International symposium on update on pelvic surgery. Genova, 5–7 Nov; 1998.
Trombetta C, Liguori G, Buttazzi L, Belgrano E. Surgical treatment of male-to-female transsexual patients: our experience. Urogynaecologia Int J. 1999;12(3):182–5.
Trombetta C, Liguori G, Bucci S, Salamè L, Garaffa G, Cova M, Belgrano E. Radiological evaluation of vaginal width and depth in male-to-female transsexuals by the use of magnetic resonance imaging. World J Urol. 2004;22(5):405–8.
Trombetta C, Liguori G, Buttazzi L, Bucci S, Belgrano E. Our technique for treatment of male-to-female transsexual patients. Br J Urol Int. 2000;86:202.
Trombetta C, Liguori G, Benvenuto S, Petrovic M, Napoli R, Umari P, Rizzo M, Zordani A. Neourethroclitoroplasty according to Petrovic. Urologia. 2011;78(4):267–73.
Trombetta C, Liguori G, Buttazzi L, Bucci S, Belgrano E. Male-to-female sex reassignment surgery (SRS): urological technique. 100th AUA Orlando 25–30 Maggio 2002. J Urol. 2002;167(4 (suppl)):149. (abstract no 597).
Stanojevic DS, Djordjevic ML, Milosevic A, Sansalone S, Slavkovic Z, Ducic S, Vujovic S, Perovic SV, Belgrade Gender Dysphoria Team. Sacrospinous ligament fixation for neovaginal prolapse prevention in male-to-female surgery. Urology. 2007;70(4):767–71.
Rezwan N, Basit AA, Andrews H. Bilateral ureteric obstruction: an unusual complication of male to female gender reassignment surgery. BMJ Case Rep. 2014;BCR-204894. https://doi.org/10.1136/bcr-2014-204894.
Savoca G, Trombetta C, De Stefani S, Raber M, Siracusano S, Belgrano E. Creazione di neovagina: presentazione di due soluzioni tecniche. In: Acts 9° Congress. S.I.A.—Ancona, 14–16 Sept “Andrologia 1995”. Bologna: Monduzzi Ed; 1995. p. 321–4.
Trombetta C, Liguori G, Bucci S, Belgrano E. Surgical therapy in transsexual patient: a multidisci-plinary approach. 18° congress of the European Association of Urology, Madrid, 12–15 March2003. Eur Urol. 2003;2(1):97.
Raza A, Byrne D, Townell N. Lower limb (well leg) compartment syndrome after urological pelvic surgery. J Urol. 2004;171:5–11.
Selvaggi G, Bellringer J. Gender reassignment surgery: an overview. Nat Rev Urol. 2011;8:274–82.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Trombetta, C. (2023). MtF Sex Reassignment Surgery: Trombetta Technique. In: Bettocchi, C., Busetto, G.M., Carrieri, G., Cormio, L. (eds) Practical Clinical Andrology. Springer, Cham. https://doi.org/10.1007/978-3-031-11701-5_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-11701-5_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11700-8
Online ISBN: 978-3-031-11701-5
eBook Packages: MedicineMedicine (R0)