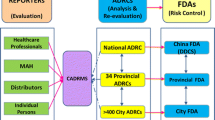
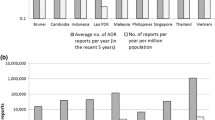
Abstract
This chapter discusses the development and reform of the pharmacovigilance system of the China National Medical Products Administration (NMPA) over the past 5 years; in particular, it reviews current approaches for pharmacovigilance for Traditional Chinese Medicinal drugs (TCMDs) in China and analyzes risk factors associated with these preparations and products, based on Adverse Drug Reaction (ADR) Information Bulletins (ADRIB) issued by the NMPA. The chapter indicates the challenges faced by the pharmacovigilance system for TCMDs in China and provides examples of possible safety concerns. Suggestions are proposed to enhance pharmacovigilance for TCMDs, including: developing signal detection techniques, active surveillance systems and pharmacoepidemiologic studies, adapted for TCMDs; exploring appropriate pharmacovigilance systems and risk-benefit evaluation models led by detecting signals from the spontaneous reporting system; establishing patient reporting to the spontaneous reporting system; addressing an international standardized system for coding for medical terminologies used in TCM and for TCMDs; strengthening surveillance of prepared slices of Chinese crude drugs (PSCCDs) and healthcare products containing PSCCDs; implementing responsibilities for marketing authorization holders in the whole life cycle for TCMDs in China.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Zhang L, Yan J, Liu X et al (2012) Pharmacovigilance practice and risk control of traditional Chinese medicine drugs in China: current status and future perspective. J Ethnopharmacol 140(3):519–525. https://doi.org/10.1016/j.jep.2012.01.058
Zhang L, Yang X, Deng Y (2009) Liver damage related to Polygonum multiflorumand its preparations: domestic literature review and analysis. China J Chin Mater Med 34(8):2414–2418
Medicines and Healthcare products Regulatory Agency (MHRA) (2014) Banned and restricted herbal ingredients. https://www.gov.uk/government/publications/list-of-banned-or-restricted-herbal-ingredients-for-medicinal-use. Accessed 5 Feb 2020
National Medical Products Administration (2019) Drug Administration Law of the People’s Republic of China (DAL). http://www.nmpa.gov.cn/WS04/CL2076/357712.html. Accessed 20 January 2020
National Medical Products Administration, National Center for ADR Monitoring (2019) Adverse drug reaction reporting system. http://www.cdr-adr.org.cn/drug_1/adrReport_1/. Accessed 5 Feb 2020
National Medical Products Administration, National Center for ADR Monitoring (2019) Direct reporting system for MAH. http://daers.adrs.org.cn/#/login?_k=z0gf96. Accessed 5 Feb 2020
National Medical Products Administration, National Center for ADR Monitoring (2011) National Center for ADR Monitoring spontaneous reporting system for healthcare professionals and marketing authorization holder. http://111.202.232.186/sso/login?service=http%3A%2F%2F111.202.232.186%2FPF%2FcasAuthUser. Accessed 5 Feb 2020
Hou Y, Li X, Wu G et al (2016) National ADR monitoring system in China. Drug Saf 39(11):1043–1051. https://doi.org/10.1007/s40264-016-0446-5
Li H, Tan N, Zhuang H et al (2020) Effect of Chinese hospital pharmacovigilance system on the reporting of adverse drug reactions in a hospital. Herald Med 39(2):265–267
Hou Y, Song H, Liu H et al (2019) Practice and discussion on active surveillance by Chinese hospital pharmacovigilance system. Chin J Pharmacovigil 16(4):212–214
The State Administration of Traditional Chinese Medicine (2011) China statistical yearbook of Chinese medicine. http://www.satcm.gov.cn/1987-2010/start.htm. Accessed 5 Feb 2020
Pharmacopoeial Commission of PRC (2017) Pharmacopoeia of the People’s Republic of China. China Medical Science Press, Beijing
The State Council of the PRC (2015) Opinions of the state council on reforming the drug and medical device evaluation and approval system. http://english.www.gov.cn/archive/state_council_gazette/2015/09/10/content_281475187020362.htm. Accessed 25 February 2020
FDA News (2017) China joins ICH as full regulatory member, pledges to implement guidelines. https://www.fdanews.com/articles/182330-china-joins-ich-as-full-regulatory-member-pledges-to-implement-guidelines. Accessed 10 Mar 2020
MOH and China Food and Drug Administration (2011) Adverse drug reaction reporting and monitoring provision. http://www.nmpa.gov.cn/WS04/CL2174/300642.html. Accessed 10 Mar 2020
National Medical Products Administration (2018) Announcement on direct report of ADR by marketing authorization holder. Accessed 25 Feb 2020
National Medical Products Administration, National Center for ADR Monitoring (2019) Announcement on on-line trial operation of electronic E2B (R3) transmission system. http://www.cdr-adr.org.cn/drug_1/zcfg_1/zcfg_zdyz/201912/t20191231_47006.html. Accessed 25 Feb 2020
National Medical Products Administration, National Center for ADR Monitoring (2019) Announcement on issuing guideline on the preparation of annual pharmacovigilance report for MAH (Interim). http://www.cdr-adr.org.cn/tzgg_home/201911/t20191129_46857.html. Accessed 25 Feb 2020
National Medical Products Administration (2019) Administrative provision for imported medicinal materials. http://www.nmpa.gov.cn/WS04/CL2138/373553.html. Accessed 25 Feb 2020
National Medical Products Administration (2018) Technical guidance for clinical trial of TCM new drug based on TCM syndrome theories. http://www.nmpa.gov.cn/WS04/CL2138/331783.html. Accessed 25 Feb 2020
Zhang L, Wong LY, He Y et al (2014) Pharmacovigilance in China: current situation, successes and challenges. Drug Saf 37(10):765–770. https://doi.org/10.1007/s40264-014-0222-3
National Medical Products Administration (2018) Guidance for clinical evaluation of drug-induced liver injury (DILI). http://www.nmpa.gov.cn/WS04/CL2138/229510.html. Accessed 25 Feb 2020
National Medical Products Administration (2006) Notification on the requirements and guidance for the printing and distribution of the formats and contents of the for the prescription of traditional Chinese medicine and natural medicine. http://www.nmpa.gov.cn/WS04/CL2196/323564.html. Accessed 25 Feb 2020
Zhang L (2018) Pharmacovigilance of herbal and traditional medicines. In: Bate A (ed) Evidence-based pharmacovigilance: clinical and quantitative aspects. Springer, New York, pp 37–65
National Medical Products Administration (2018) Notification on publishing the work programme for strengthening the quality of prepared slices of Chinese crude drugs. http://www.nmpa.gov.cn/WS04/CL2196/330076.html. Accessed 25 Feb 2020
National Medical Products Administration (2020) Notification on procedures and requirements for keeping records of provincial norm for the PSCCMs’ process. http://www.nmpa.gov.cn/WS04/CL2196/374149.html. Accessed 25 Feb 2020
National Medical Products Administration (2020) Annual report for national ADR monitoring. http://www.nmpa.gov.cn/WS04/CL2155/376451.html. Accessed 16 May 2020
Zhang L, Yang X, Cao L (2005) Pondering on current status and development of monitoring on adverse reaction of traditional Chinese medicine in China. Chin J Integr Trad West Med 25(7):581–584
Zhang L, Ye Z, Ji S (2012) Safety monitoring of traditional Chinese medicine injections: the current status and risk management. World Sci Technol Modern Tradit Chin Med Mater Medica 12(6):845–850
China Food and Drug Administration (2009) Notification on doing well in the reevaluation of TCM injections. http://www.nmpa.gov.cn/WS04/CL2196/323740.html. Accessed 20 Mar 2020
China Food and Drug Administration (2010) Notice on publication the 7 principals for technical guideline on TCM injection safety re-evaluation concerning production process, quality control clinical study and so on. http://www.ccpie.org/cn/yjxx/yphzp/webinfo/2010/10/1481297440376509.htm. Accessed 20 Mar 2020
National Medical Products Administration (2008) Notification on further strengthening the production, management and clinical rational use of TCMI. http://www.nmpa.gov.cn/WS04/CL2079/333373.html. Accessed 20 Mar 2020
Yan YY, Yang YH, Wang WW et al (2017) Post-marketing safety surveillance of the Salvia miltiorrhiza depside salt for infusion: a real world study. PLoS One 12(1):e0170182. https://doi.org/10.1371/journal.pone.0170182
Li XX, Zhuo L, Zhang Y et al (2019) The incidence and risk factors for adverse drug reactions related to Tanreqing injection: a large population-based study in China. Front Pharmacol 10:1523. https://doi.org/10.3389/fphar.2019.01523
China Food and Drug Administration (2013) Notification on promoting key monitoring of pharmaceuticals manufacturing industries (draft for comments). http://www.nmpa.gov.cn/WS04/CL2101/228805.html. Accessed 20 Mar 2020
Li X, Li H, Deng J et al (2018) Active pharmacovigilance in China: recent development and future perspectives. Eur J Clin Pharmacol 74(7):863–871. https://doi.org/10.1007/s00228-018-2455-z
Zhang L, Yang X (2004) Cause analysis and countermeasure of ADR caused by traditional Chinese medicine. China J Tradit Chin Med Pharm 19(8):496–498
Zhang L, Yang X (2009) Pharmacovigilance idea should be introduced sufficiently into the safety monitoring and evaluation process of Chinese drugs. Chin J Integr Trad West Med 29(9):843–846
Zhu F, Yang M, Chen W et al (2015) Correlation between toxicity and content of Curculiginis Rhizoma and Epimedii Folium with different compatible proportion. Chin J Exp Tradit Med Formulae 21(5):175–177. https://doi.org/10.13422/j.cnki.syfjx.2015050175
WHO Regional Office for South-East Asia (2009) The use of herbal medicines in primary health care. WHO Regional Office for South-East Asia. https://apps.who.int/iris/handle/10665/206476
Zhang L, Wang G, Lin R (2009) Analysis and discussion on the pharmacovigilance information and measures of risk control in China (1). Chin Pharm Affairs 23(8):735–739
Zhang L, Wang G, Lin R (2009) Analysis and discussion on the pharmacovigilance information and measures of risk control in China (2). Chin Pharm Affairs 23(9):853–856
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zhang, L., Yang, T. (2022). Pharmacovigilance for Traditional Chinese Medicinal Drugs in China. In: Barnes, J. (eds) Pharmacovigilance for Herbal and Traditional Medicines. Adis, Cham. https://doi.org/10.1007/978-3-031-07275-8_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-07275-8_23
Published:
Publisher Name: Adis, Cham
Print ISBN: 978-3-031-07273-4
Online ISBN: 978-3-031-07275-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)