Abstract
Man-made persistent pollutants (such as PCBs, pesticides and trace metals) reach aquatic organisms through the food chains. Pollutants are ingested and assimilated by smaller organisms, and their concentration in tissues increases from prey to predators. Being at the top of the food chains, marine mammals accumulate some of the highest environmental contaminant levels of all wildlife. They are good sentinel species for monitoring long-term environmental pollution. Exposure to contaminants may have large consequences, both on an individual and a population level. The prevalence and severity of diseases of aquatic wildlife has recently increased in many species. Scientists use new methods to understand how pollutants affect the immune system of marine mammals. Learning about contaminants may also contribute to our understanding of outbreaks of infectious diseases in marine mammals.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Bioaccumulation and biomagnification
- Environmental contaminants
- Necropsy
- Organic pollutants
- Toxins
- Trace elements
-
Understand how different pollutants affect marine mammals.
-
Learn how biomagnification and bioaccumulation affect marine life.
-
Learn how to monitor wildlife health by post-mortem examinations.
1 Introduction
Many organic and inorganic chemicals are manufactured by humans and end up in our oceans. As stated by marine scientist C. M. Reddy of the Woods Hole Oceanographic Institution in 2008: ‘During the course of the 20th century, the planet became and is now chemically different from any previous time’. These chemicals are synthesised or formed by natural processes through human activities.
Many different types of human-made compounds cause problems for wildlife. There are toxic persistent organic pollutants (POPs), which are industrial compounds, and toxic trace elements, previously known as heavy metals. POPs and trace elements occur in nature in increased abundance because of human activities. Previously, POPs were used as pesticides, industrial chemicals, solvents and pharmaceuticals. They are chemically stable and do not easily degrade through natural processes.
The biological half-life describes how quickly a chemical compound (including medications) is reduced to half of its initial concentration in, for example, body tissues. Usually, POPs dissolve well in lipids (but not in water) and are therefore soluble in fatty tissues. They are poorly metabolised (and therefore have a long biological half-life). Because they easily bind to the surface of solid particles, they are easily ingested and assimilated as a part of the animal’s nutrition. Because of all these features, POPs are prone to dietary accumulation—so-called bioaccumulation, or biomagnification—in fatty tissues, with potential adverse health impacts.
With more humans inhabiting coastal regions, the health of oceans becomes a more important issue for everyone. At higher temperatures, POPs volatize and reach the atmosphere, where they can travel long distances before they are finally re-deposited. Therefore POPs may accumulate also in areas far from their emission, like Antarctica. Hence, environmental contamination by POPs is extensive, and they will often remain in the environment for decades (◘ Fig. 1).
Marine mammals can be used as early indicators for negative trends and impacts linked to anthropogenic (human-made) activities. Such sentinel species will also permit us to better characterise and potentially manage negative impact on human and animal health because of us polluting our oceans.
Bioaccumulation of contaminants
Organisms take in toxic chemicals through contaminated food, water, and/or air. The gastrointestinal tract concentrates ingested stable and hydrophobic (low affinity to water) chemicals. When these substances are stored in fatty body tissues, they increase in concentration inside the organism. This is known as bioaccumulation.
Biomagnification trough the food web
Biomagnification (also known as bioamplification) causes toxic compounds to be found at higher concentrations in tissues of organisms belonging to a higher level in the food chain. With each step upwards the food web, the concentration of pollutants increases up to ten times in animal tissues. The toxic compounds are transferred from smaller to larger organism, from prey to predator. Because the compounds are easily assimilated but have a long biological half-life, they accumulate within the tissues. The higher the trophic level an animal feeds at, the more chemicals build up within the body. The amount of increase depends on the biological half-life of the substance, and how easily it is assimilated, metabolised or excreted by the organism.
Marine mammals and contaminants
Marine mammals accumulate high levels of toxic POPs and trace elements in their tissues (blubber, liver, hair) because of their unique biological and ecological features:
-
Marine mammals have extensive fat stores in which lipophilic contaminants (easily dissolved in fat) accumulate.
-
They are at the top (or close to the top) of marine food webs.
-
They are homeothermic (warm-blooded) animals, eating large quantities of food containing pollutants.
-
Marine mammals have a long lifespan, and pollutants accumulate over time.
2 The use of marine mammals as bioindicators
The distribution of chemical pollutants in the marine environment is not homogeneous, and a considerable variation of concentrations may occur regionally and temporally. It may therefore be difficult to assess the full environmental impact of pollutants. As a supplement to measurements of the contaminant levels at different sites, bioindicators can be used to monitor pollution. Bioindicators are living organisms used to assess the levels of pollutants in the ecosystems where they live. Marine mammals are highly suitable bioindicators of the marine environment. Due to their position at the top of the food webs, their long lifespan and the long biological half-time of pollutants, marine mammals accumulate high levels of different toxic chemicals.
The interest in studying contaminants in marine mammals was boosted by past large-scale die-offs or impaired reproduction abilities of pinnipeds and cetaceans living in polluted regions, and the discovery of high contaminant levels in these animals. In many cases, morbillivirus infections were the primary cause of disease outbreaks in these animals. Famous examples are the harbour seal mass mortalities in the North Sea in 1988 and 2002. Scientists investigate if environmental pollution plays a role in mass mortalities, as toxic chemicals may suppress the immune system.
3 How harmful are pollutants?
Several chemical pollutants, such as DDT, PCB, TBT (Tributyltin) and metallic trace elements, are endocrine disrupting chemicals (EDCs), meaning they affect the hormonal system. Hormonal disruptions can influence many systems in the body, such as the endocrine system itself, reproduction (cause birth defects and developmental disorders), immune cell generation, as well as causing tumours.
Endocrine disrupting chemicals may interfere with the hormone synthesis in the endocrine gland, the hormone transport or the hormone’s metabolism and excretion within its target cells. Since many hormones regulate reproductive functions, exposure to EDCs often has negative consequences for reproductive health. Embryos, foetuses and newborns are especially vulnerable to EDCs, causing future problems in brain function, immunity, metabolism and reproductive abilities.
Furthermore, EDCs can alter the synthesis of steroid hormones and have adverse effects on the mechanisms of molecules operated by genes and proteins. Pollutants most likely promote disease and mortality by supressing the immune system. Alterations to energy metabolism can lead to obesity, diabetes, and cardiovascular disease development, as well as have adverse effects on the immune system. Additionally, synergistic effects between various contaminants may amplify the toxicity of different chemicals. Interactions with environmental factors, for example, pathogens, starvation, and climate change (changes in water temperature, pH and salinity) could also amplify the contaminant toxicity and bioavailability.
Trace element pollutants, such as mercury, cadmium, and lead, have especially high cell toxicity and accumulation characteristics. Mercury and PCBs are potent immunosuppressants in terrestrial and aquatic animals, altering host resistance to disease. Animals with high contamination levels seem to be more susceptible to diseases than animals with lower toxin burdens. Since EDC effects are usually subtle and more chronic than acute, it is often difficult to link certain health impairments to specific exposures.
Example
A well-studied case of contamination effects comes from the St. Lawrence River Estuary beluga whale (Delphinapterus leucas) population in Canada. These belugas live at the southernmost limit of the species range, are geographically isolated from other populations and were listed as endangered in 2014 by the Committee on the Status of Endangered Wildlife in Canada. Pollution and human disturbance have reduced food resources and caused habitat degradation, which seem to contribute to the decline of this species.
The St. Lawrence River Estuary receives water from one of the world’s most industrialised regions. The belugas are heavily contaminated by trace elements, PCB, DDT and PAHs (polycyclic aromatic hydrocarbons). Exposure to highly toxic discharges from local aluminium smelters led to elevated contaminant levels in the tissue of the belugas and had toxicological effects. From studies made between 1983 and 2006, 16% of 175 stranded belugas had at least one terminal cancerous tumour. Some of the cancer types found in belugas are related to the presence of PAHs, suggesting that these compounds are involved in the cause of cancer in the SLE belugas.
These effects of pollution are not restricted to belugas only. The human population living by the St. Lawrence River Estuary is also suffering from higher cancer rates than other Canadians.
Contamination is considered a serious threat to the SLE beluga population recovery. Despite reductions in the discharge of some toxic chemicals, pollutant concentrations in tissues do not decrease quickly. Some effects on contaminants may first develop 20 years after exposure. The belugas could be affected by contaminants for many decades to come.
4 Post-mortem examinations
A post-mortem examination is the examination of a dead animal. For humans it is also called an autopsy, whereas for any non-human animal it is called a necropsy. The aim is to determine the cause of death, how and when the animal died and to obtain a better understanding of how diseases spread. Often, a proper health assessment of living marine mammals is not possible. Therefore, we rely on post-mortem examinations of animals that are stranded or caught in fishing nets to obtain information of animal health and diseases.
Post-mortem examinations elucidate the cause of death and common diseases of the species investigated. We also learn about transmissible pathogens between humans and animals (so-called zoonoses) and the influence of anthropogenic activities on wildlife. Additionally, necropsies are the main source of samples used for toxicological analysis, which helps researchers to associate contaminant loads with clinical observations and pathologic health impairments. This helps us to identify how different pollutants and their concentrations affect the health of marine mammals.
A full post-mortem examination includes:
-
Measurements of body size (length and girths), weight and colouration
-
Macroscopical examination of all organs with the naked eye
-
Histological assessment of all organs using microscopy
-
Bacteriology (investigating bacteria and their connection to disease)
-
Mycology (study of fungal infections)
-
Parasitology (study of parasites and their interactions with the host)
-
Virology (study of viruses and their connection to disease)
-
Toxicology (study of adverse effects of chemical substances)
-
Serology (detect antibodies caused by infections in serum)
-
Age determination
-
Genetics
For a small animal such as a harbour porpoise (Phocoena phocoena), a necropsy lasts 2-4 h, depending on the animal’s decomposition status. For a larger whale, necropsy may last for several days, depending on available work force and technical equipment, as well as the stranding location.
Some extra examinations can be undertaken depending on financial means, which species is investigated, and under which circumstances it was found. Additional examinations may include immunohistology (the microscopic study of tissues with the aid of antibodies that bind to tissue components and reveal their presence), electron microscopy, bone density measurements, and analysis of stomach content and reproductive organs. Such examinations are complex and time consuming, and analysis may take several months.
The cause of disease and death may be determined, and the general health status of the individual can be assessed. This may also inform the health status on the population and the habitats frequented by the animal. However, it is not always possible to determine a cause of death. Some cases remain unsolved even after a thorough post-mortem examination.
Disease can be a major cause of population decline in marine mammals, and the reasons for many stranding events remain poorly understood. New sequencing technology, virological and microbiological studies can identify pathogens and diseases and help in surveillance.
For watching a short video about the necroscopy of a harbour porpoise, see: (► https://wissen.hannover.de/en/Institutions/University-of-Veterinary-Medicine-Hannover/Looking-into-Animals-Necropsies-at-the-TiHo).
5 Common diseases of marine mammals
Marine mammals, just like any animal, can suffer from different kinds of diseases. These can affect their health adversely, cause pain, distress and even death, and therefore have negative implications for the entire population.
Diseases can be caused by different reasons:
-
Infectious diseases by viral, bacterial, parasitic and fungal infections
-
Non-infectious diseases by toxins (from pollutants or algae), starvation or predation
For any cause of disease there is often secondary bacterial and parasitic infections, most common in the lungs.
A high load of parasites (e.g. pulmonary roundworms, gastrointestinal nematodes and tapeworms, liver and gastric flukes), pneumonia, acute traumata (from bleedings or fractures), chronic disease and direct anthropogenic impacts are common for stranded marine mammals. Impaired hearing, as well as disruption of the hormonal and immunological system, can also have severe, adverse impacts on individual health.
Chemical pollution may play a role in the pathogenicity (the ability of an organism to cause disease) of several types of diseases of marine mammals. Pathogenic viruses have been associated with meningitis, bronchopneumonia, skin diseases and changes in the reproductive system. Different influenza A virus strains have caused at least five larger die-offs of seals in the past 40 years. There is a risk that these diseases can be zoonotic (being able to transfer to and infect humans).
A deteriorated health status from an increased pollutant burden can lead to devastating viral epidemics. Huge morbillivirus die-offs were caused by the Phocine Distemper Virus (PDV) in 1988–1989, 1990–1991, and 2002 in harbour seals in the North Sea and Kattegat, and by a dolphin morbillivirus in 1990–1991 in striped dolphins (Stenella coeruleoalba) in Mediterranean waters. The epizootic (disease event in an animal population, analogous to an epidemic in humans) PDV outbreaks killed thousands of animals, and the disease susceptibility of the infected individuals was probably caused by contaminant-induced immunosuppression.
Exposure to a mixture of different PCBs decreases the immune response and increases the risk for virus infections. If other environmental factors also favour virus replication and their rapid spread, combined effects may lead to epizootic outbreaks. Furthermore, PCB exposure indicated a contaminant-related disruption of hormone function of free-ranging harbour seals and harbour porpoises, leading to reduced reproduction.
Contamination in the Baltic Sea from the 1970s and 1980s
Elevated POP levels in Baltic grey seals (Haliocherus grypus) and ringed seals (Pusa hispida) in the 1970s and 1980s were linked to reproductive failure and several different tissue lesions, causing the so-called ‘Baltic Seal Disease Complex’. Affected seals had smaller thyroid glands (responsible for secretion of hormones regulating growth and development) and enlarged adrenal glands (producing a variety of hormones, including adrenaline, cortisol, and sexual hormones). The uterus experienced stenosis (abnormal narrowing), occlusion and tumours, resulting in impaired reproduction. There were also claw lesions, loss of bone structure in the skull and reduced bone mineral density.
High concentrations of organochlorine are associated with a low mineral density in trabecular bone (porous, internal skeletal bone tissue found at the ends of long bones, in the pelvic bones, ribs, skull, and vertebrae). Measurements of bone mineral density provide insights into the bones’ health and can determine the risk for fractures. Severe intestinal ulcers and increased parasitic burdens have also been associated with high loads of DDT and PCB.
Zoonotic diseases can be dangerous for people encountering dead or sick marine mammals. People working with marine mammals have the highest risk of acquiring zoonotic diseases. Therefore, marine mammal researchers, rehabilitators, trainers, veterinarians and volunteers must be extra careful. People encountering captive or wild marine mammals during, for example, vacations should also be careful. Zoonoses caused by bacteria, fungi or viruses are easily transmissible. Luckily, the majority of transmissions from marine mammals to humans have only resulted in localised skin infections that can resolve spontaneously or with appropriate medical therapy. However, some zoonoses can lead to life-threatening systemic diseases. When encountering dead or alive marine mammals, always keep a safe distance and call appropriate authorities.
Growing amounts of anthropogenic influences and utilisation of the marine ecosystem constantly increase the pressure on marine mammals, their habitat and the associated stress and disease risk. Marine mammals may face more infectious diseases in the future, enhanced by the prevalence of contaminants in the environment and in the food chains.
Current topics of chemical pollution and diseases of marine mammals research
Chemical pollution affecting marine mammals is an important topic for current research, since it includes many unanswered questions. Even though there is knowledge on intake pathways, bioaccumulation and biomagnification, as well as on geographical and temporal contaminant trends, data is lacking on the implications on marine mammals.
There have been many questions regarding the impact of plastics and other debris on the marine fauna, including marine mammals. This includes the transport of contaminants to coastal ecosystems (persistent, bioaccumulative and toxic chemicals attach to plastics) and impacts of macro as well as micro plastics. It is still unclear if ingested plastics add significantly to the existing contamination load. Plastics are nowadays such an important topic, so that this book dedicates a whole chapter to them.
Many studies are conducted on diverse species and different known and measurable pollutants. However, this does not mean that all potential pollutants are known and tested for. There is still a wide grey zone of unknowns, including newly emerging contaminants, their accumulation within the environment, impacts they have on different species, and potential human exposure.
Many of the current studies on cetaceans focus on organochlorine contaminants and their reproductive implications, particularly in endangered species and subspecies, since they are especially vulnerable. This focus may change over time with new analytical methods. Orcas are on top of the trophic food chain and a very long-living species. Thus, they are highly susceptible to contaminant biomagnification and consequential reproductive impairment, since immunosuppression can have detrimental effects on offspring (embryos during pregnancy, calves receiving large amounts of contaminants through nursing) and population survival.
Stable isotopes and biomarkers are used to assess contaminant exposure as a feeding ecology tool and to assess the bodily response to environmental pollutants. Stable isotopes are non-radioactive variations of chemical elements. Measuring and analysing their distribution, amounts and proportions in samples can be used to trace the origin, history, source, chemical interactions, and carbon and nitrogen cycles of the studied sample. Biomarkers are characteristic biologic traits that can indicate normal or pathogenic processes associated with stress (e.g. environmental pollution or diseases) within organs, cells, genes, gene products, or hormones of the studied organism. Since the primary reason for wildlife contaminant exposure is their feeding ecology, effective new tools for diet determination and habitat use are key elements of many eco-toxicological studies.
Another important topic for current research is the individual and population health effects of oil exposure on marine mammals. An example is the extremely long and large oil spill caused by Deepwater Horizon in the Gulf of Mexico in 2010. Many cetaceans have succumbed to different disease complexes associated with the spill and suffered from reproductive failure and abortions. Establishing a link between the massive oil spill and its effect on wildlife is crucial for preventing future disasters and establishing appropriate management plans for similar human activities.
We need to consider effects of both older pollutants such as PCBs, which are banned in Europe and North America but are still long-lasting in the food chains, as well as newer chemical pollutants. We need to improve pollutant management and design effective conservation measures. It is also crucial to develop sampling and analysis methods for new contaminants, to generate new mitigation measures to prevent further contamination, as well as develop functional cleaning methods. Last but not least, we need to tackle these issues on a global scale to prevent further entries of pollutants into the environment.
Apart from PCBs and trace metals, pharmaceuticals (including human and veterinary drugs) are another important class of contaminants entering the world’s waterways. Thousands of tons of pharmacologically active substances are used annually worldwide. Unfortunately, they receive relatively little attention as possible environmental pollutants. Up to 90% of consumed pharmaceuticals can be excreted unchanged, while environmental bacterial action can convert utilised metabolites into active drug compounds. Additionally, unused medicines are often disposed through the sewage system, and many pharmaceuticals are only incompletely eliminated at sewage treatment plants. The possible effects of the presence of drugs in aquatic systems are widely unknown.
A major concern has so far been that antibiotics found in effluent sewage may cause increased resistance among bacterial populations exposed to these drugs. There are currently several studies looking for multi-drug-resistant bacteria in marine life. However, most aquatic organisms are continually exposed to a whole range of different substances. Especially in coastal regions, pharmaceuticals may suppress the immune response and hormone production of aquatic organisms.
Several studies have shown that many aquatic animals are affected by marine environment polluting drugs: Oysters from two different bays in Canada contained traces of medications such as antibiotics, antihistamines (used for allergy treatment) and pain relievers. ‘Intersex’ fish, with both male and female reproductive organs caused by endocrine disrupters, have been found worldwide. Scientists believe that artificial hormones from birth control pills may contribute to this problem. Antidepressant and antianxiety medications are also found globally in the environment. They accumulate in wildlife tissues, and their potential to disrupt normal biological systems and behaviours is extensive.
Many aquatic organisms spend their entire lives in polluted environments, affecting their immune system, feeding habits, behaviour, metabolism, and movement patterns. Prozac (a common antidepressant used for the treatment of depression) causes shrimp (Echinogammarus marinus) to leave their natural, hidden habitat and head towards more luminous locations, making them vulnerable to predators. Small amounts of cocaine can have adverse health effects on critically endangered European eels (Anguilla anguilla). Cocaine-exposed eels were hyperactive and suffered from muscle damage. These problems do not end in our rivers or oceans: When we eat seafood, the pharmaceuticals and contaminants return to our bodies, affecting our physiology and starting a new vicious circle.
6 Teaching materials
Exercise 5.1: How can different types of pollution affect marine mammals?
Have a class discussion:
-
Identify sources of, and solutions to air pollution.
-
Identify sources of, and solutions to water pollution.
-
What can everyone do to fight environmental pollution?
-
List at least ten ways to avoid pollution. Maybe some students already implement some of these ways and others are not. Can the students support one another to increase our efforts to reduce pollution?
Tip
Let the students collect all discarded items they find on their way to school. Discuss the potential the objects have to harm wildlife.
Some activities anybody can do to help reduce pollution:
-
Walk or ride a bicycle instead of driving in a car.
-
Turn off lights and unplug electronic devices when not needed.
-
Switch to reusable water bottles, mugs and bags.
-
Use eco-friendly and energy-efficient products whenever possible.
-
Buy locally grown and produced food products.
-
Use soap bars and other minimal/zero waste products.
-
Reduce, reuse and recycle as often as you can.
-
Properly discard expired medications.
-
Plant trees, grow your own fruits and vegetables.
Be sure to share facts that will emphasise the importance of your activities and why you are doing them. For example, when you are recycling, explain that recycling just one glass bottle reduces air pollution by 20 percent and causes 50 percent less water pollution compared to making a brand-new bottle.
Exercise 5.2: Mussel filtration
Organisms get rid of contaminants through catabolism and excretion, but how does the ocean get rid of pollutants?
In this experiment, we will demonstrate the important role of mussels as waste collectors within aquatic ecosystems by showing their ability to clear water.
Marine mussels are bivalve (they have two hard shells) molluscs. There are among other species, blue mussels, oysters, and clams, which all improve water quality and contribute to healthy marine habitats. They play an important role in aquatic ecosystems. Mussels are filter feeders. They draw in seawater and filter out phytoplankton and sediments. A video showing the anatomy of a mussel can be found at ► https://www.youtube.com/watch?v=gZKSFBj-FqU.
On this 3D animation ► https://www.youtube.com/watch?v=7KekxV78gns you can observe filter and particulate organic matter filtration by a blue mussel (Mytilus edulis). The animation shows the path of water (blue) and associated food (orange) in the mussel. Siphoned material is either transferred to the mouth for digestion or sloughs off the gills and exits via the ventral margin of the shell. Digested material is used both as fuel for various life processes and excreted as faeces. The amount and rate of particulate matter removed from the water column and subsequent deposition of waste depends on species, size, water temperature and particle concentration.
Marine mussels function as bioindicators of marine pollution. As sedentary suspension feeders, mussels remove a variety of materials from the water column. These materials include pollutants that can be assimilated and bioaccumulate in their tissues.
Required materials
-
Two aquaria, container or buckets full of seawater (use rain or lake water if freshwater species are used).
-
Aquarium pump (you don’t need a strong one; it can be a very simple, cheap model) or compressor.
-
Living blue mussels. Commercially available from supermarkets; if possible, collect them with the students from a nearby harbour (if blue mussels cannot be obtained, freshwater mussels from a lake or aquarist stores are also an option).
-
A planktonic algae mix (from a nearby lake, or cultivated beforehand).
-
Food colouring.
-
A video recording device with a timer (e.g. a smartphone).
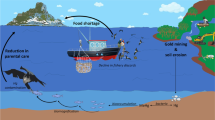
Illustration of PCB bioaccumulation in the marine food chain. Pollution and the related biomagnification within organisms are a global problem. The numbers associated with the shown media/species refer to a constructed mean contaminant concentration within these, to show the significant effect of biomagnification. © Guillaume Bolterys
Tasks
-
1.
Collect the blue mussels and keep them in a well-aired aquarium around 15–20 °C. Arrange the second aquarium next to the first one in a similar way, with water but without mussels.
-
2.
Pour the mix of planktonic algae equally into both aquariums and observe how fast the mussels are able to clear the water. Try to film the process or take a picture before and after the clearing of the seawater. If you have a photometer, you can also take measurements of the differences in light reflection between the two aquaria. ◘ Figure 2 shows the result of the experiment.
-
3.
Put the food colouring into water of both aquaria, let it spread and see what happens.
-
4.
Write down your observations and times. Draw conclusions and discuss them within your group.
(► Exercise 5.2) Mussel filtration experiment. The upper picture shows two aquaria with sea water, the right one also contains mussels. The bottom picture shows that after 11 min, the mussels have cleared the water completely, while the tank without mussels remains unchanged
After the experiment, the living mussels should be returned to their place of origin, or they can be kept in suitable aquarium; or a dissection can be done to view the food colouring deposition within the mussel by colouration of the organs. While the mussels are alive, you can observe the ciliary movements at the level of the gills with a binocular microscope. You should, however, consider how to humanely kill the mussels prior to dissection, for animal welfare reasons. This can be done by boiling, by bubbling carbon dioxide or adding a 20–30% concentration of magnesium sulphate into the water. Please do NOT use formaldehyde or alcohol for euthanasia purposes.
You can also watch a recording of the whole mussel clearing experiment ► (Video S1).
Exercise 5.3: Bioaccumulation: The hidden dangers in the food web
The processes of bioaccumulation and biomagnification are connected. How can we visualise a better understanding of these concepts?
With this simple experiment, students can observe how contaminants accumulate and magnify in different organisms within the food chain. Through ingestion with their prey, chemicals move up through the food chain. Bioaccumulation means that even when the initial level of chemicals was low, the concentration accumulates in organisms higher up the food web, increasing their toxic potential.
Required materials
-
1 ‘shaker’ cup
-
9 small cups (corresponding to small animal, e.g. shrimp)
-
3 medium cups (corresponding to medium fish, e.g. cod)
-
1 large cup (predator, e.g. harbour porpoise)
-
20 items of the same colour (e.g. blue sweets) as plankton
-
10 items of the same colour (e.g. red sweets) as plankton with DDT attached
Tasks
Place all 30 items in the ‘shaker’ to represent the population of primary producers and give it a good shake. Record the amounts of DDT (number of, for example, red sweets) per producer (for example, 10 contaminants per 30 producers gives a total of 1/3)
-
1.
Simulate sand lance eating some of the plankton by closing your eyes and randomly removing 3 items from the ‘shaker’. Place them into one of the small ‘sand lance’ cups and repeat this for the remaining eight small cups. Record the amount of DDT in each sand lance (see ◘ Fig. 3).
-
2.
Now, simulate the cod eating two sand lances. Empty the contents of two randomly chosen ‘sand lance’ cups into one of the medium ‘cod’ cups. Repeat for the remaining two cod cups. Record the amount of DDT in each ‘cod’.
-
3.
Finally, simulate the porpoise eating cod. One porpoise needs to consume two cods. Empty the contents of two randomly chosen ‘cod’ cups into the large ‘porpoise’ cup. Record the amount of DDT in the porpoise.
-
4.
Place all items back into the ‘shaker’ cup and repeat the experiment two more times. Then calculate the average amount of DDT for each organism from all three trials.
(► Exercise 5.3) Top: game requirements (differently sized cups, a ‘shaker’ and ‘plankton’ items of two different colours); Bottom: playing the game—simulation of bioaccumulation by emptying the contents of two randomly chosen small ‘sand lance’ cups into one of the medium ‘cod’ cups
Draw conclusions to marine life by using the following questions:
-
Comparing all three trials, which organism contained the highest concentration of DDT?
-
What happened to the amount of DDT per organism as it moved up the food chain?
-
Why is DDT harmful to marine mammals?
-
Name other organisms besides porpoises that you would expect to have high concentrations of DDT.
-
If the porpoise population decreases due to contamination effects, which other populations of marine mammals would be affected?
-
Which of the following types of sea food would be the safest to eat, concerning their content of pollutants? List them in order and explain your answer.
Herring, Squid, Salmon, Mackerel, Orca, Mussels, Shark, Cod, Tuna
Exercise 5.4: Oil spill clean-up (Part 1)
Imagine an oil spill into a body of water—what methods or materials could be used to clean up the oil?
The ocean has been subject to many different small oil leakages and large oil spill disasters with major environmental impacts. There are chronic spills: The Niger Delta is polluted by over 13 million barrels of crude oil, with an average yearly spill of 240,000 barrels. More commonly, we hear about wrecked oil cargo ships such as the ‘Amoco Cadiz’ crude oil carrier spill in France in 1978 and the ‘Exxon Valdez’ oil tanker spill in Alaska in 1989. In this exercise, you will model an oil spill, look at the impact of oil on seabirds and test different materials for cleaning up the spill. This experiment will help you understand why an oil spill is an environmental catastrophe and a difficult task to deal with.
Required materials
-
Baking dish
-
Hot and cold tap water
-
Blue food colouring
-
Vegetable oil
-
Pure cocoa powder
-
Teaspoon
-
Stir rods
-
Beaker
-
Sorbents (paper towel, kitchen towel, different textile fabrics, cotton balls, sponges, Styrofoam cup, straw or hay, shredded wheat, garden peat moss, etc.)
-
Liquid dishwashing detergent
-
Forceps
-
Clean, dry feathers
-
Three bowls or basins
Tasks
First, you have to prepare your clean water and ‘crude oil’.
-
1.
To prepare the ‘ocean’ fill the baking dish with cold tap water to within 2 cm of rim, add the food dye and stir it until it has a nice colour. Let the solution settle.
-
2.
To simulate crude oil use 3 tablespoons of vegetable oil and thoroughly mix in 2 tbsp. of cocoa powder. (This experiment also works with regular vegetable oil, but the effect is clearer and more realistic with the thicker oil-cocoa mix.)
-
3.
To contaminate fresh water, pour the simulated crude oil very slowly directly onto the surface of the freshwater dish. Be cautious: if you pour too quickly, the experiment will not work—in this case, start over!
-
What happened to the oil when you dropped it on the ocean? Record your observations and explain them.
-
-
4.
To test the sorbents each student should choose ~3 sorbents to test, so that all available options are being tested. Before starting, write a hypothesis on how the different sorbents you selected will clean up oil and which of them will work best.
Test the sorbents one at a time and record your observations thoroughly.
-
How much oil did the sorbent clean up?
-
Is the sorbent fast or slow absorbing?
-
Does the sorbent pick up water too?
-
Does the clean sorbent sink or float?
-
Does it change if oil-coated?
-
Which sorbent worked the fastest?
-
Which one worked the best overall?
-
How would you pick up oil-contaminated sorbents in a real oil spill in fresh water/the ocean?
-
How would you dispose the tons of toxic oil-contaminated material from a real oil spill?
-
-
5.
Now add 2–3 drops of detergent to the oil-contaminated freshwater. Describe what happens. Would detergent be a reasonable tool to use in a real oil spill? Discuss the pros and cons with your classmates.
Look back at your original hypothesis and write a concluding statement that recommends materials and methods for cleaning up oil spills based on your findings.
Oil spill clean-up (Part 2)
How are animals affected by oil and related clean-up methods?
-
6.
To look at the way oil affects bird feathers, you will try out different clean-up methods to find out which ones work best (depending on how much detergent was used in step 5., a new oiled water preparation might be necessary for this part). Before starting, discuss how different animals are affected by an oil spill and what happens to birds and their feathers in particular.
-
What is the function of feathers for birds?
-
Which water bird species can you think of being affected by oil spills?
-
-
7.
Choose some feathers and dip them in the oil to imitate what happens when a bird lands on an oil slick. What happened to the feathers? How do you think this might affect a water bird? Write down your observations and thoughts.
-
8.
Now try three methods of cleaning feathers. Therefore, we need to set up 3 washing stations. One with cold water, one with hot water and one with warm water and detergent. Choose a washing technique for your feathers and use the same method at each station.
-
(a)
Cold water washing: try washing some of the oiled feathers in cold water. Write down your observations.
-
(b)
Hot water washing: try washing some of the oiled feathers in hot water. Write down your observations.
-
(c)
Washing with detergent: try washing some of the oiled feathers in the warm soapy water. Write down your observations.
-
(a)
-
9.
Which method would be best to clean oily birds? Write a final statement that discusses how oil spills affect birds, what the best cleaning method would be and incorporate your own findings.
Oil spill clean-up (Part 3): Group discussion
Discuss the following points within your class and perform some online research yourself on the issue:
-
Birds may ingest oil while trying to preen the oil from their feathers—how does this effect their health and survival chances?
-
Aquatic animals are usually extremely sensitive creatures—is only the ingestion of oil dangerous or are there other problems related to external toxin exposure?
-
Every oil spill is different, because the kinds of oil that are used vary widely—what could be a difference between a crude oil spill and a spill of highly toxic oils such as diesel or jet fuel?
-
Washing birds within 8–24 h of capture is advantageous in order to reduce absorption of toxins through the skin and possible resultant liver and kidney damage. However, cleaning (restraint and handling) is a very stressful procedure for a wild bird—should birds be cleaned immediately after capture or should they not be washed until their physical and mental condition is stable (such that they are likely to survive the procedure) even if that increases the chances of intoxication through toxin absorption?
-
Why is it so important to make sure the bird is thoroughly rinsed and definitely clean after the washing procedure?
-
Example answers:
-
The ingestion of oil leads to intoxication and potential interference with internal organ functionality, decreasing their health and survival chances.
-
Toxin resorption through the skin and oil contamination of fur and feathers prevent thermoregulation or swimming ability; animals can get stuck in an oil blanket.
-
Crude oil is less processed and hence often less toxic than highly refined oils, but might be more difficult to clean off.
-
The animal should always be stabilised prior to a washing procedure, but also be cleaned as soon as possible to prevent further intoxication damage. Distressed animals require calming before washing, which can take several days.
-
Residual oil and/or detergent will interfere with waterproofing and insulation of the bird.
Exercise 5.5: Greenhouse gases in the ocean
It is often mentioned that the ocean functions as a huge CO2 trap, which is of major importance for climate regulation, but how does it do that?
The ocean absorbs gases from the atmosphere and releases them again. Thus, the world’s oceans have a major influence on the world climate and also absorb a lot of airborne contaminants. Gases like the greenhouse gas carbon dioxide or other pollutants can dissolve in the water, just like salt does. How much gas the water can absorb depends on various factors that can easily be tried out with this little experiment.
Required materials
-
0.5 l bottle (transparent)
-
Bowl
-
Small funnel
-
Tap water (optional: food colouring)
-
Effervescent tablets (e.g. Alka-Seltzer tablet)
-
Permanent marker
Tasks
Fill half of the bowl and the bottle to the brim with warm tap water. For a nicer optic, you can dye the water with food colouring.
-
1.
Place the funnel into the bottle and carefully position everything upside down in the bowl (bottle opening facing down). Put an effervescent tablet under the funnel and let it dissolve. During this process, carbon dioxide is produced and CO2 bubbles fizz into the bottle. The more CO2 is produced, the more water gets pushed out of the bottle. Once the tablet is dissolved, indicate the lower edge of the resulting gas bubble with the marker.
-
2.
Repeat the experiment with cold water. Is the new marker in the same place as the first one?
-
3.
What will happen if you put a second effervescent tablet under the funnel? Will the bubble within the bottle be twice as big, less or more than twice as big as in the first trail?
-
4.
Discuss your results and gather some explanations.
What does this mean for other pollutants and for aquatic animals?
If you are unsure, perform an online search on sea temperature increase and its effects on ecosystems.
Explanation
In this experiment, we have to discern the invisible from the visible gas bubbles: not all the produced gas arrives at the top of the bottle, because—invisible to us—a certain portion is absorbed by the water. The gas basically ‘dissolves’ in it. The ability of the water to absorb gases depends on the temperature and the amount of gas already dissolved in the water: the colder the water, the more gas can be absorbed, resulting in a smaller gas bubble within the bottle (Step 2). The second effervescent tablet (Step 3) then dissolves in water, which already contains a lot of gas from the first trail (it is almost ‘saturated’). Therefore, a much larger proportion of gas directly fizzes into the bottle now.
In the past, water in the world’s oceans contained relatively little carbon dioxide, and large quantities of greenhouse gases could therefore pass from the air into the water at the ocean surface. Meanwhile, our oceans slowly begin to warm due to climate change caused by carbon dioxide. Due to both effects (saturation and temperature increase), the oceans are less able to absorb this gas. It is a vicious cycle.
Suggested reading
Desforges J-P, Hall A, McConnell B, Rosing-Asvid A, Barber JL, Brownlow A, De Guise S, Eulaers I, Jepson PD, Letcher RJ, Levin M, Ross PS, Samarra F, Víkingson G, Sonne C, Dietz R 2018. Predicting global killer whale population collapse from PCB pollution. Science 361:1373–6. https://doi.org/10.1126/science.aat1953.
Fabbri E, Franzellitti S 2016. Human pharmaceuticals in the marine environment: focus on exposure and biological effects in animal species. Environ Toxicol Chem 35:799–812. https://doi.org/10.1002/etc.3131.
Fossi MC, Panti C 2018. Marine mammal ecotoxicology: impacts of multiple stressors on population health. London: Elsevier, Academic Press ISBN: 9780128122501.
Gulland FMD, Dierauf LA, Whitman KL 2018. CRC Handbook of Marine Mammal Medicine. 3rd ed. Boca Ranton, FL: CRC Press. ISBN 9781498796873.
Jepson PD, Deaville R, Barber JL, Aguilar A, Borrell A, Murphy S, Barry J, Brownlow A, Barnett J, Berrow S, Cunningham AA, Davison NJ, ten Doeschate M, Esteban R, Ferreira M, Foote AD, Genov T, Giménez J, Loveridge J, Llavona Á, Martin V, Maxwell DL, Papachlimitzou A, Penrose R, Perkins MW, Smith B, de Stephanis R, Tregenza N, Verborgh P, Fernandez A, Law RJ 2016. PCB pollution continues to impact populations of orcas and other dolphins in European waters. Scientific Rep 6:18573. https://doi.org/10.1038/srep18573.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
(MP4 100434 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Reckendorf, A., Siebert, U., Parmentier, E., Das, K. (2023). Chemical Pollution and Diseases of Marine Mammals. In: Brennecke, D., Knickmeier, K., Pawliczka, I., Siebert, U., Wahlberg, M. (eds) Marine Mammals. Springer, Cham. https://doi.org/10.1007/978-3-031-06836-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-06836-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06835-5
Online ISBN: 978-3-031-06836-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)