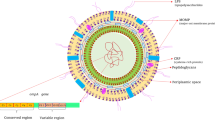
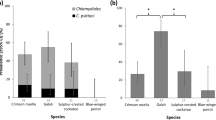
Abstract
Historically, the first documented cases of infections by chlamydiae involved humans with contact to psittacine birds. While birds have remained the main source of zoonotic transmission until now, the spectrum of chlamydial zoonoses has broadened in recent decades.
In the present chapter, we summarize current knowledge on etiology, pathology, epidemiology, and genomic markers of zoonotic chlamydial infections. In particular, Chlamydia (C.) psittaci, the agent of avian chlamydiosis, is continuing to affect human individuals in contact with birds. Clinical signs can range from flu-like to those of severe systemic illness. C. abortus, a pathogen causing enzootic abortion in small ruminants, was repeatedly shown to be responsible for cases of human abortion. C. caviae, an agent of ocular disease in guinea pigs, is known to have caused conjunctivitis in guinea pig owners. Also C. felis, which can cause acute and chronic conjunctivitis in cats, has a zoonotic potential and was associated with ocular disease in contact persons.
We outline the main characteristics of the agents’ animal reservoirs, describe transmission routes, and summarize recent reports on outbreaks and individual cases of human infections by Chlamydia spp. The relatively low number of officially notified cases is probably due to underdiagnosis, since C. psittaci and other chlamydiae are usually not part of routine diagnosis in human medicine. As research in the past decades has led to the extension of the genus Chlamydia to 14 species and 4 taxa at Candidatus rank, the scope of zoonotic agents can be expected to rise in the future.
Similar content being viewed by others
References
Akter R, Sansom FM, El-Hage CM, Gilkerson JR, Legione AR, Devlin JM (2021) A 25-year retrospective study of Chlamydia psittaci in association with equine reproductive loss in Australia. J Med Microbiol 70(2):001284
Anstey S, Lizárraga D, Nyari S, Chalmers G, Carrick J, Chicken C, Jenkins C, Perkins N, Timms P, Jelocnik M (2021) Epidemiology of Chlamydia psittaci infections in pregnant Thoroughbred mares and foals. Vet J. 273:105683
Arenas-Valls N, Chacon S, Perez A, Del Pozo R (2017) Atypical Chlamydia psittaci pneumonia. Four related cases. Arch Bronconeumol 53:277–279
Baker JA (1942) A virus obtained from a pneumonia of cats and its possible relation to the cause of atypical pneumonia in man. Science 96:475–476
Baker JA (1944) A virus causing pneumonia in cats and producing elementary bodies. J Exp Med 79:159–172
Barros E (1929) Epidemia de psittacosis. Diario médico 152
Bart M, Guscetti F, Zurbriggen A, Pospischil A, Schiller I (2000) Feline infectious pneumonia: a short literature review and a retrospective immunohistological study on the involvement of Chlamydia spp. and distemper virus. Vet J 159:220–230
Baumann S, Gurtner C, Marti H, Borel N (2020) Detection of Chlamydia species in 2 cases of equine abortion in Switzerland: a retrospective study from 2000 to 2018. J Vet Diagn Invest 32:542–548
Beeckman DS, Vanrompay DC (2009) Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect 15:11–17
Borel N, Polkinghorne A, Pospischil A (2018) A review on chlamydial diseases in animals: still a challenge for pathologists? Vet Pathol 55:374–390
Branley J, Bachmann NL, Jelocnik M, Myers GS, Polkinghorne A (2016) Australian human and parrot Chlamydia psittaci strains cluster within the highly virulent 6BC clade of this important zoonotic pathogen. Sci Rep 6:30019
Bressan M, Rampazzo A, Kuratli J, Marti H, Pesch T, Borel N (2021) Occurrence of Chlamydiaceae and Chlamydia felis pmp9 typing in conjunctival and rectal samples of Swiss stray and pet cats. Pathogens 10
Browning GF (2004) Is Chlamydophila felis a significant zoonotic pathogen? Aust Vet J 82:695–696
Burnard D, Polkinghorne A (2016) Chlamydial infections in wildlife-conservation threats and/or reservoirs of ‘spill-over’ infections? Vet Microbiol 196:78–84
Burt SA, Roring RE, Heijne M (2018) Chlamydia psittaci and C. avium in feral pigeon (Columba livia domestica) droppings in two cities in the Netherlands. Vet Q 38:63–66
Buxton D (1986) Potential danger to pregnant women of Chlamydia psittaci from sheep. Vet. Rec 510–511
Buxton D, Barlow RM, Finlayson J, Anderson IE, Mackellar A (1990) Observations on the pathogenesis of Chlamydia psittaci infection of pregnant sheep. J Comp Pathol 102:221–237
Buxton D, Anderson IE, Longbottom D, Livingstone M, Wattegedera S, Entrican G (2002) Ovine chlamydial abortion: characterization of the inflammatory immune response in placental tissues. J Comp Pathol 127:133–141
Caspe SG, Palarea-Albaladejo J, Underwood C, Livingstone M, Wattegedera SR, Milne E, Sargison ND, Chianini F, Longbottom D (2021) Distribution and severity of placental lesions caused by the Chlamydia abortus 1B vaccine strain in vaccinated ewes. Pathogens 10
Chan J, Doyle B, Branley J, Sheppeard V, Gabor M, Viney K, Quinn H, Janover O, McCready M, Heller J (2017) An outbreak of psittacosis at a veterinary school demonstrating a novel source of infection. One Health 3:29–33
Chen X, Cao K, Wei Y, Qian Y, Liang J, Dong D, Tang J, Zhu Z, Gu Q, Yu W (2020) Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection 48:535–542
Chisu V, Foxi C, Masu G, B DA, Masala G (2020) Detection of potentially pathogenic bacteria from Ixodes ricinus carried by pets in Tuscany, Italy. Vet Rec Open 7:e000395
De Puysseleyr K, De Puysseleyr L, Dhondt H, Geens T, Braeckman L, Morre SA, Cox E, Vanrompay D (2014a) Evaluation of the presence and zoonotic transmission of Chlamydia suis in a pig slaughterhouse. BMC Infect Dis 14:560
De Puysseleyr K, De Puysseleyr L, Geldhof J, Cox E, Vanrompay D (2014b) Development and validation of a real-time PCR for Chlamydia suis diagnosis in swine and humans. PLoS One 9:e96704
De Puysseleyr L, De Puysseleyr K, Braeckman L, Morre SA, Cox E, Vanrompay D (2017) Assessment of Chlamydia suis infection in pig farmers. Transbound Emerg Dis 64:826–833
Dean D, Rothschild J, Ruettger A, Kandel RP, Sachse K (2013) Zoonotic Chlamydiaceae species associated with trachoma, Nepal. Emerg Infect Dis 19:1948–1955
Deschuyffeleer TP, Tyberghien LF, Dickx VL, Geens T, Saelen JM, Vanrompay DC, Braeckman LA (2012) Risk assessment and management of Chlamydia psittaci in poultry processing plants. Ann Occup Hyg 56:340–349
Di Francesco A, Donati M, Mazzeo C, Battelli G, Piva S, Cevenini R, Baldelli R (2006) Feline chlamydiosis: a seroepidemiological investigation of human beings with and without contact with cats. Vet Rec 159:778–779
Dickx V, Geens T, Deschuyffeleer T, Tyberghien L, Harkinezhad T, Beeckman DS, Braeckman L, Vanrompay D (2010) Chlamydophila psittaci zoonotic risk assessment in a chicken and Turkey slaughterhouse. J Clin Microbiol 48:3244–3250
Donati M, Laroucau K, Guerrini A, Balboni A, Salvatore D, Catelli E, Lupini C, Levi A, Di Francesco A (2018) Chlamydiosis in backyard chickens (Gallus gallus) in Italy. Vector Borne Zoonotic Dis 18:222–225
Dujardin-Beaumetz G (1893) Enquete sur des cri de pneumonie infectieuse paraissant avoir été occassionés par des perruches. Bull. Conseil. Hyg. publ. salubrité Départ. Seine April 1893
Dumke R, Schnee C, Pletz MW, Rupp J, Jacobs E, Sachse K, Rohde G, Capnetz Study G (2015) Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011–2012. Emerg Infect Dis 21:426–434
Essig A, Longbottom D (2015) Chlamydia abortus: new aspects of infectious abortion in sheep and potential risk for pregnant women. Curr Clin Microbiol Rep 2:22–34
Ferreira VL, Silva MV, Bassetti BR, Pellini ACG, Raso TF (2017) Intersectoral action for health: preventing psittacosis spread after one reported case. Epidemiol Infect 145:2263–2268
Fischer N, Rohde H, Indenbirken D, Gunther T, Reumann K, Lutgehetmann M, Meyer T, Kluge S, Aepfelbacher M, Alawi M, Grundhoff A (2014) Rapid metagenomic diagnostics for suspected outbreak of severe pneumonia. Emerg Infect Dis 20:1072–1075
Gaede W, Reckling KF, Dresenkamp B, Kenklies S, Schubert E, Noack U, Irmscher HM, Ludwig C, Hotzel H, Sachse K (2008) Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health 55:184–188
Gaede W, Reckling KF, Schliephake A, Missal D, Hotzel H, Sachse K (2010) Detection of Chlamydophila caviae and Streptococcus equi subsp. zooepidemicus in horses with signs of rhinitis and conjunctivitis. Vet Microbiol 142:440–444
Gruffydd-Jones T, Addie D, Belak S, Boucraut-Baralon C, Egberink H, Frymus T, Hartmann K, Hosie MJ, Lloret A, Lutz H, Marsilio F, Pennisi MG, Radford AD, Thiry E, Truyen U, Horzinek MC (2009) Chlamydophila felis infection. ABCD guidelines on prevention and management. J Feline Med Surg 11:605–609
Haagen E, Mauer G (1938) Über eine auf den Menschen übertragbare Viruskrankheit bei Sturmmöwen und ihre Beziehung zur Psittakose. Zbl Bakteriologie, Parasitenkunde und Infektionskrankheiten 143, I. Abt., Originale, 81–88
Haas WH, Swaan CM, Meijer A, Neve G, Buchholz U, Beer M, van Steenbergen JE, Krause G (2007) A Dutch case of atypical pneumonia after culling of H5N1 positive ducks in Bavaria was found infected with Chlamydophila psittaci. Euro Surveill 12(E071129):071123
Hahn DL (1999) Chlamydia pneumoniae, asthma, and COPD: what is the evidence? Ann Allergy Asthma Immunol 83:271–288, 291; quiz 291–272
Halanova M, Sulinova Z, Cislakova L, Trbolova A, Palenik L, Weissova T, Halan M, Kalinova Z, Holickova M (2011) Chlamydophila felis in cats--are the stray cats dangerous source of infection? Zoonoses Public Health 58:519–522
Hartley JC, Stevenson S, Robinson AJ, Littlewood JD, Carder C, Cartledge J, Clark C, Ridgway GL (2001) Conjunctivitis due to Chlamydophila felis (Chlamydia psittaci feline pneumonitis agent) acquired from a cat: Case report with molecular characterization of isolates from the patient and cat. J Infection 43:7–11
Hegler C (1930) Psittacosis – clinical experience with humans [in German]. Dt Tierärztl Wschr 38:677–681
Heijne M, van der Goot JA, Fijten H, van der Giessen JW, Kuijt E, Maassen CBM, van Roon A, Wit B, Koets AP, Roest HIJ (2018) A cross sectional study on Dutch layer farms to investigate the prevalence and potential risk factors for different Chlamydia species. PLoS One 13:e0190774
Henning K, Sachse K, Sting R (2000) Nachweis von Chlamydien bei einem Stutenabort [Demonstration of Chlamydia from an equine abortion]. In Dtsch Tierarztl Wochenschr pp 49–52
Herrmann B, Persson H, Jensen JK, Joensen HD, Klint M, Olsen B (2006) Chlamydophila psittaci in fulmars, the Faroe Islands. Emerg Infect Dis 12:330–332
Hinton DG, Shipley A, Galvin JW, Harkin JT, Brunton RA (1993) Chlamydiosis in workers at a duck farm and processing plant. Aust Vet J 70:174–176
Hogerwerf L, DE Gier B, Baan B, Hoek WVANDER (2017) Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect 145:3096–3105
Holzer M, Barf LM, Lamkiewicz K, Vorimore F, Lataretu M, Favaroni A, Schnee C, Laroucau K, Marz M, Sachse K (2020) Comparative genome analysis of 33 Chlamydia strains reveals characteristic features of Chlamydia psittaci and closely related species. Pathogens 9
Hoover EA, Kahn DE, Langloss JM (1978) Experimentally induced feline chlamydial infection (feline pneumonitis). Am J Vet Res 39:541–547
Hughes C, Maharg P, Rosario P, Herrell M, Bratt D, Salgado J, Howard D (1997) Possible nosocomial transmission of psittacosis. Infect Control Hosp Epidemiol 18:165–168
Hulin V, Oger S, Vorimore F, Aaziz R, de Barbeyrac B, Berruchon J, Sachse K, Laroucau K (2015) Host preference and zoonotic potential of Chlamydia psittaci and C. gallinacea in poultry. Pathog Dis 73:1–11
Hyde SR, Benirschke K (1997) Gestational psittacosis: case report and literature review. Mod Pathol 10:602–607
Ito I, Ishida T, Mishima M, Osawa M, Arita M, Hashimoto T, Kishimoto T (2002) Familial cases of psittacosis: possible person-to-person transmission. Intern Med 41:580–583
Jelocnik M, Branley J, Heller J, Raidal S, Alderson S, Galea F, Gabor M, Polkinghorne A (2017) Multilocus sequence typing identifies an avian-like Chlamydia psittaci strain involved in equine placentitis and associated with subsequent human psittacosis. Emerg Microbes Infect 6:e7
Joseph SJ, Marti H, Didelot X, Castillo-Ramirez S, Read TD, Dean D (2015) Chlamydiaceae genomics reveals interspecies admixture and the recent evolution of Chlamydia abortus infecting lower mammalian species and humans. Genome Biol Evol 7:3070–3084
Kaleta EF, Taday EM (2003) Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathol 32:435–461
Kalmar ID, Dicxk V, Dossche L, Vanrompay D (2014) Zoonotic infection with chlamydia psittaci at an avian refuge Centre. Vet J 199:300–302
Kieckens E, Van den Broeck L, Van Gils M, Morre S, Vanrompay D (2018) Co-occurrence of Chlamydia suis DNA and Chlamydia suis-specific antibodies in the human eye. Vector Borne Zoonotic Dis 18:677–682
Knittler MR, Sachse K (2014) Chlamydia psittaci: update on an underestimated zoonotic agent (Minireview). FEMS Pathog Dis 73. https://doi.org/10.1093/femspd/ftu1007
Krautwald-Junghanns ME, Stolze J, Schmidt V, Bohme J, Sachse K, Cramer K (2013) Efficacy of doxycycline for treatment of chlamydiosis in flocks of racing and fancy pigeons. Tierarztliche Praxis. Ausgabe K, Kleintiere/Heimtiere 41:392–398
Lagae S, Kalmar I, Laroucau K, Vorimore F, Vanrompay D (2014) Emerging Chlamydia psittaci infections in chickens and examination of transmission to humans. J Med Microbiol 63:399–407
Laroucau K, Aaziz R, Meurice L, Servas V, Chossat I, Royer H, de Barbeyrac B, Vaillant V, Moyen JL, Meziani F, Sachse K, Rolland P (2015) Outbreak of psittacosis in a group of women exposed to Chlamydia psittaci-infected chickens. Euro Surveill 20:pii: 21155
Laroucau K, de Barbeyrac B, Vorimore F, Clerc M, Bertin C, Harkinezhad T, Verminnen K, Obeniche F, Capek I, Bebear C, Durand B, Zanella G, Vanrompay D, Garin-Bastuji B, Sachse K (2009) Chlamydial infections in duck farms associated with human cases of psittacosis in France. Vet Microbiol 135:82–89
Laroucau K, Vorimore F, Aaziz R, Solmonson L, Hsia RC, Bavoil PM, Fach P, Holzer M, Wuenschmann A, Sachse K (2019) Chlamydia buteonis, a new Chlamydia species isolated from a red-shouldered hawk. Syst Appl Microbiol 42:125997
Laroucau K, Ortega N, Vorimore F, Aaziz R, Mitura A, Szymanska-Czerwinska M, Cicerol M, Salinas J, Sachse K, Caro MR (2020) Detection of a novel Chlamydia species in captive spur-thighed tortoises (Testudo graeca) in southeastern Spain and proposal of Candidatus Chlamydia testudinis. Syst Appl Microbiol 43:126071
Lepore J (2009) It’s spreading – outbreaks, media scares, and the parrot panic of 1930. The New Yorker American Chronicles June 1
Li L, Luther M, Macklin K, Pugh D, Li J, Zhang J, Roberts J, Kaltenboeck B, Wang C (2017) Chlamydia gallinacea: a widespread emerging Chlamydia agent with zoonotic potential in backyard poultry. Epidemiol Infect 145:2701–2703
Livingstone M, Wheelhouse N, Maley SW, Longbottom D (2009) Molecular detection of Chlamydophila abortus in post-abortion sheep at oestrus and subsequent lambing. Vet Microbiol 135:134–141
Longbottom D, Coulter LJ (2003) Animal chlamydioses and zoonotic implications. J Comp Pathol 128:217–244
Longbottom D, Livingstone M, Maley S, van der Zon A, Rocchi M, Wilson K, Wheelhouse N, Dagleish M, Aitchison K, Wattegedera S, Nath M, Entrican G, Buxton D (2013) Intranasal infection with Chlamydia abortus induces dose-dependent latency and abortion in sheep. PLoS One 8:e57950
Longbottom D, Sait M, Livingstone M, Laroucau K, Sachse K, Harris SR, Thomson NR, Seth-Smith HMB (2018) Genomic evidence that the live Chlamydia abortus vaccine strain 1B is not attenuated and has the potential to cause disease. Vaccine 36:3593–3598
Longbottom D, Livingstone M, Ribeca P, Beeckman DSA, van der Ende A, Pannekoek Y, Vanrompay D (2021) Whole genome de novo sequencing and comparative genomic analyses suggests that Chlamydia psittaci strain 84/2334 should be reclassified as Chlamydia abortus species. BMC Genomics 22:159
Low HC, Powell CC, Veir JK, Hawley JR, Lappin MR (2007) Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res 68:643–648
Lutz-Wohlgroth L, Becker A, Brugnera E, Huat ZL, Zimmermann D, Grimm F, Haessig M, Greub G, Kaps S, Spiess B, Pospischil A, Vaughan L (2006) Chlamydiales in guinea-pigs and their zoonotic potential. J Vet Med A Physiol Pathol Clin Med 53:185–193
Magnino S, Haag-Wackernagel D, Geigenfeind I, Helmecke S, Dovc A, Prukner-Radovcic E, Residbegovic E, Ilieski V, Laroucau K, Donati M, Martinov S, Kaleta EF (2009) Chlamydial infections in feral pigeons in Europe: review of data and focus on public health implications. Vet Microbiol 135:54–67
Maley SW, Livingstone M, Rodger SM, Longbottom D, Buxton D (2009) Identification of Chlamydophila abortus and the development of lesions in placental tissues of experimentally infected sheep. Vet Microbiol 135:122–127
McDonald M, Willett BJ, Jarrett O, Addie DD (1998) A comparison of DNA amplification, isolation and serology for the detection of Chlamydia psittaci infection in cats. Vet Rec 143:97–101
McGovern OL, Kobayashi M, Shaw KA, Szablewski C, Gabel J, Holsinger C, Drenzek C, Brennan S, Milucky J, Farrar JL, Wolff BJ, Benitez AJ, Thurman KA, Diaz MH, Winchell JM, Schrag S (2021) Use of real-time PCR for Chlamydia psittaci detection in human specimens during an outbreak of psittacosis – Georgia and Virginia, 2018. MMWR Morb Mortal Wkly Rep 70:505–509
McGuigan CC, McIntyre PG, Templeton K (2012) Psittacosis outbreak in Tayside, Scotland, December 2011 to February 2012. Eur Secur 17:20186
Meijer A, Brandenburg A, de Vries J, Beentjes J, Roholl P, Dercksen D (2004) Chlamydophila abortus infection in a pregnant woman associated with indirect contact with infected goats. Eur J Clin Microbiol Infect Dis 23:487–490
Meyer KF, Eddie B (1935) Avian psittacosis. J Bacteriol 29:67
Morange A (1895) De la psittacose, ou infection spéciale déterminée par des perruches. Thesis, Fac. de Mèdecine, Paris
Mount DT, Bigazzi PE, Barron AL (1973) Experimental genital infection of male guinea pigs with the agent of guinea pig inclusion conjunctivitis and transmission to females. Infect Immun 8:925–930
Murray ES (1964) Guinea pig inclusion conjunctivitis virus. I. Isolation and identification as a member of the psittacosis-lymphogranuloma-trachoma group. J Infect Dis 114:1–12
Myers GS, Mathews SA, Eppinger M, Mitchell C, O’Brien KK, White OR, Benahmed F, Brunham RC, Read TD, Ravel J, Bavoil PM, Timms P (2009) Evidence that human Chlamydia pneumoniae was zoonotically acquired. J Bacteriol 191:7225–7233
Ornelas-Eusebio E, Garcia-Espinosa G, Vorimore F, Aaziz R, Durand B, Laroucau K, Zanella G (2020) Cross-sectional study on Chlamydiaceae prevalence and associated risk factors on commercial and backyard poultry farms in Mexico. Prev Vet Med 176:104922
Ortega N, Caro MR, Gallego MC, Murcia-Belmonte A, Alvarez D, Del Rio L, Cuello F, Buendia AJ, Salinas J (2015) Isolation of Chlamydia abortus from a laboratory worker diagnosed with atypical pneumonia. Ir Vet J 69:8
Page LA, Grimes JE (1978) Avian chlamydiosis (ornithosis). In: Hofstad MS, Barnes HJ, Calnek BW, Yoder WM, Yoder HW Jr (eds) Diseases of poultry, 8th edn. Iowa State University Press, Ames, IA, pp 283–308
Pantchev A, Sting R, Bauerfeind R, Tyczka J, Sachse K (2010) Detection of all Chlamydophila and Chlamydia spp. of veterinary interest using species-specific real-time PCR assays. Comp Immunol Microbiol Infect Dis 33:473–484
Polkinghorne A, Greub G (2017) A new equine and zoonotic threat emerges from an old avian pathogen, Chlamydia psittaci. Clin Microbiol Infect 23:693–694
Polkinghorne A, Borel N, Heijne M, Pannekoek Y (2019) New evidence for domesticated animals as reservoirs of Chlamydia-associated community-acquired pneumonia. Clin Microbiol Infect 25:131–132
Pospischil A, Thoma R, Hilbe M, Grest P, Zimmermann D, Gebbers JO (2002) Abortion in humans by Chlamydophila abortus (Chlamydia psittaci serovar 1). In Schweiz. Arch. Tierheilk pp 463–466
Ramakers BP, Heijne M, Lie N, Le TN, van Vliet M, Claessen VPJ, Tolsma PJP, De Rosa M, Roest HIJ, Vanrompay D, Heddema ER, Schneeberger P, Hermans MHA (2017) Zoonotic Chlamydia caviae presenting as community-acquired pneumonia. N Engl J Med 377:992–994
Rampazzo A, Appino S, Pregel P, Tarducci A, Zini E, Biolatti B (2003) Prevalence of Chlamydophila felis and feline herpesvirus 1 in cats with conjunctivitis in northern Italy. J Vet Intern Med 17:799–807
Read TD, Joseph SJ, Didelot X, Liang B, Patel L, Dean D (2013) Comparative analysis of Chlamydia psittaci genomes reveals the recent emergence of a pathogenic lineage with a broad host range. MBio 4
Rehn M, Ringberg H, Runehagen A, Herrmann B, Olsen B, Petersson AC, Hjertqvist M, Kuhlmann-Berenzon S, Wallensten A (2013) Unusual increase of psittacosis in southern Sweden linked to wild bird exposure, January to April 2013. Euro Surveill 18:20478
Reinhold P, Sachse K, Kaltenboeck B (2011) Chlamydiaceae in cattle: commensals, trigger organisms, or pathogens? Vet J 189:257–267
Ritter J (1879) Über Pneumotyphus, eine Hausepidemie in Uster. Dtsch Arch Klin Med 25:53
Roberts W, Grist NR, Giroud P (1967) Human abortion associated with infection by ovine abortion agent. Br Med J 4:37
Rodolakis A, Yousef Mohamad K (2010) Zoonotic potential of Chlamydophila. Vet Microbiol 140:382–391
Sachse K, Bavoil PM, Kaltenboeck B, Stephens RS, Kuo CC, Rossello-Mora R, Horn M (2015a) Emendation of the family Chlamydiaceae: proposal of a single genus, Chlamydia, to include all currently recognized species. Syst Appl Microbiol 38:99–103
Sachse K, Borel N (2020) Recent advances in epidemiology, pathology and immunology of veterinary chlamydiae (Chapter 17). Caister Academic Press, pp 403–428. https://doi.org/10.21775/9781912530281
Sachse K, Laroucau K, Hotzel H, Schubert E, Ehricht R, Slickers P (2008) Genotyping of Chlamydophila psittaci using a new DNA microarray assay based on sequence analysis of ompA genes. BMC Microbiol 8:63
Sachse K, Laroucau K, Riege K, Wehner S, Dilcher M, Creasy HH, Weidmann M, Myers G, Vorimore F, Vicari N, Magnino S, Liebler-Tenorio E, Ruettger A, Bavoil PM, Hufert FT, Rossello-Mora R, Marz M (2014) Evidence for the existence of two new members of the family Chlamydiaceae and proposal of Chlamydia avium sp. nov. and Chlamydia gallinacea sp. nov. Syst Appl Microbiol 37:79–88
Sachse K, Laroucau K, Vanrompay D (2015b) Avian Chlamydiosis. Curr Clin Microbiol Rep 2:10–21
Sachse K, Ruettger A (2015) Rapid microarray-based genotyping of Chlamydia spp. strains from clinical tissue samples. Methods Mol Biol 1247:391–400
Sammin D, Markey B, Bassett H, Buxton D (2009) The ovine placenta and placentitis-A review. Vet Microbiol 135:90–97
Sanderson H, Vasquez M, Killion H, Vance M, Sondgeroth K, Fox J (2021) Fatal Chlamydia psittaci infection in a domestic kitten. J Vet Diagn Invest 33:101–103
Sandoz KM, Rockey DD (2010) Antibiotic resistance in Chlamydiae. Future Microbiol 5:1427–1442
Schachter J, Ostler HB, Meyer KF (1969) Human infection with the agent of feline pneumonitis. Lancet 1:1063–1065
Schautteet K, Vanrompay D (2011) Chlamydiaceae infections in pig. Vet Res 42:29
Schmal-Filius E, Nedorost N, Weissenbacher-Lang C, Weissenbock H (2020) A retrospective study on the presence of selected infectious agents in lung samples of cats with pneumonia. Acta Vet Hung 68:275–284
Seth-Smith HMB, Buso LS, Livingstone M, Sait M, Harris SR, Aitchison KD, Vretou E, Siarkou VI, Laroucau K, Sachse K, Longbottom D, Thomson NR (2017) European Chlamydia abortus livestock isolate genomes reveal unusual stability and limited diversity, reflected in geographical signatures. BMC Genomics 18:344
Shewen PE, Povey RC, Wilson MR (1978) Case report. Feline chlamydial infection. Can Vet J 19:289–292
Staub E, Marti H, Biondi R, Levi A, Donati M, Leonard CA, Ley SD, Pillonel T, Greub G, Seth-Smith HMB, Borel N (2018) Novel Chlamydia species isolated from snakes are temperature-sensitive and exhibit decreased susceptibility to azithromycin. Sci Rep 8:5660
Suchland RJ, Sandoz KM, Jeffrey BM, Stamm WE, Rockey DD (2009) Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob Agents Chemother 53:4604–4611
Sykes JE (2005) Feline chlamydiosis. Clin Tech Small Anim Pract 20:129–134
Sykes JE, Anderson A, Studdert VP, Browning GF (1999a) Prevalence of feline Chlamydia psittaci and feline Herpesvirus 1 in cat with upper respiratory tract disease. In J Vet Intern Med 153–162
Sykes JE, Studdert VP, Browning GF (1999b) Comparison of the polymerase chain reaction and culture for the detection of feline Chlamydia psittaci in untreated and doxycycline-treated experimentally infected cats. J Vet Intern Med 13:146–152
Szeredi L, Hotzel H, Sachse K (2005) High prevalence of chlamydial (Chlamydophila psittaci) infection in fetal membranes of aborted equine fetuses. Vet Res Commun 29(Suppl 1):37–49
Szymanska-Czerwinska M, Mitura A, Niemczuk K, Zareba K, Jodelko A, Pluta A, Scharf S, Vitek B, Aaziz R, Vorimore F, Laroucau K, Schnee C (2017) Dissemination and genetic diversity of chlamydial agents in Polish wildfowl: isolation and molecular characterisation of avian Chlamydia abortus strains. PLoS One 12:e0174599
Taise SM (2013) Avian Chlamydiosis – retrospective analysis of an epidemic with zoonotic potential, from its first description to the present [in German]. Thesis, University of Giessen, Germany. http://geb.unigiessen.de/geb/volltexte/2014/10664/pdf/TaiseSabine_12013_10612_10609.pdf
Taylor-Brown A, Bachmann NL, Borel N, Polkinghorne A (2016) Culture-independent genomic characterisation of Candidatus Chlamydia sanzinia, a novel uncultivated bacterium infecting snakes. BMC Genomics 17:710
Taylor-Brown A, Polkinghorne A (2017) New and emerging chlamydial infections of creatures great and small. New Microbes New Infect 18:28–33
Taylor KA, Durrheim D, Heller J, O’Rourke B, Hope K, Merritt T, Freeman P, Chicken C, Carrick J, Branley J, Massey P (2018) Equine chlamydiosis-An emerging infectious disease requiring a one health surveillance approach. Zoonoses Public Health 65:218–221
TerWee J, Sabara M, Kokjohn K, Sandbulte J, Frenchick P, Dreier KJ (1998) Characterization of the systemic disease and ocular signs induced by experimental infection with Chlamydia psittaci in cats. Vet Microbiol 59:259–281
Van Droogenbroeck C, Beeckman DS, Verminnen K, Marien M, Nauwynck H, Boesinghe Lde T, Vanrompay D (2009) Simultaneous zoonotic transmission of Chlamydophila psittaci genotypes D, F and E/B to a veterinary scientist. Vet Microbiol 135:78–81
Van Grootveld R, Bilsen MP, Boelsums TL, Heddema ER, Groeneveld GH, Gooskens J, de Boer MGJ (2018) Chlamydia caviae causing community-acquired pneumonia: An emerging zoonosis. Vector Borne Zoonotic Dis
Van Loock M, Geens T, De Smit L, Nauwynck H, Van Empel P, Naylor C, Hafez HM, Goddeeris BM, Vanrompay D (2005) Key role of Chlamydophila psittaci on Belgian Turkey farms in association with other respiratory pathogens. Vet Microbiol 107:91–101
Vanrompay D (2013) Avian Chlamydiosis. In: Diseases of poultry, Bacterial diseases, vol Section III, 13th edn. Wiley- Blackwell Publishing
Vanrompay D, Butaye P, Sayada C, Ducatelle R, Haesebrouck F (1997) Characterization of avian Chlamydia psittaci strains using omp1 restriction mapping and serovar-specific monoclonal antibodies. Res Microbiol 148:327–333
Vanrompay D, Harkinezhad T, van de Walle M, Beeckman D, van Droogenbroeck C, Verminnen K, Leten R, Martel A, Cauwerts K (2007) Chlamydophila psittaci transmission from pet birds to humans. Emerg Infect Dis 13:1108–1110
Vorimore F, Holzer M, Liebler-Tenorio EM, Barf LM, Delannoy S, Vittecoq M, Wedlarski R, Lecu A, Scharf S, Blanchard Y, Fach P, Hsia RC, Bavoil PM, Rossello-Mora R, Laroucau K, Sachse K (2021) Evidence for the existence of a new genus Chlamydiifrater gen. nov. inside the family Chlamydiaceae with two new species isolated from flamingo (Phoenicopterus roseus): Chlamydiifrater phoenicopteri sp. nov. and Chlamydiifrater volucris sp. nov. Syst Appl Microbiol 44:126200
Vorimore F, Hsia RC, Huot-Creasy H, Bastian S, Deruyter L, Passet A, Sachse K, Bavoil P, Myers G, Laroucau K (2013) Isolation of a new Chlamydia species from the Feral Sacred Ibis (Threskiornis aethiopicus): Chlamydia ibidis. PLoS One 8:e74823
Vorimore F, Thebault A, Poisson S, Cleva D, Robineau J, de Barbeyrac B, Durand B, Laroucau K (2015) Chlamydia psittaci in ducks: a hidden health risk for poultry workers. Pathog Dis 73:1–9
Wachendorfer G, Luthgen W (1974) Chlortetracycline impregnated food-pellets for the prophylaxis and therapy of psittacosis/ornithosis in psittacines and pigeons. Avian Pathol 3:105–114
Walder G, Meusburger H, Hotzel H, Oehme A, Neunteufel W, Dierich MP, Wurzner R (2003) Chlamydophila abortus pelvic inflammatory disease. In Emerg Infect Dis 1642–1644
Walder G, Hotzel H, Brezinka C, Gritsch W, Tauber R, Wurzner R, Ploner F (2005) An unusual cause of sepsis during pregnancy: recognizing infection with Chlamydophila abortus. Obstet Gynecol 106:1215–1217
Wallensten A, Fredlund H, Runehagen A (2014) Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January-February 2013. Euro Surveill 19
Wang H, Jensen JK, Olsson A, Vorimore F, Aaziz R, Guy L, Ellstrom P, Laroucau K, Herrmann B (2020) Chlamydia psittaci in fulmars on the Faroe Islands: a causative link to south American psittacines eight decades after a severe epidemic. Microbes Infect 22:356–359
Wattegedera SR, Livingstone M, Maley S, Rocchi M, Lee S, Pang Y, Wheelhouse NM, Aitchison K, Palarea-Albaladejo J, Buxton D, Longbottom D, Entrican G (2020) Defining immune correlates during latent and active chlamydial infection in sheep. Vet Res 51:75
Wills JM, Grufydd-Jones TJ, Richmond SJ, Gaskell RM, Bourne FJ (1987) Effect of vaccination on feline Chlamydia psittaci infection. Infect Immun 55:2653–2657
Winkle S (2000) The history of the parrot fever epidemic [in German: Die Seuchengeschichte der Papageienkrankheit]. Hamb Arztebl 2:50–58
Wons J, Meiller R, Bergua A, Bogdan C, Geissdorfer W (2017) Follicular conjunctivitis due to Chlamydia felis-Case report, review of the literature and improved molecular diagnostics. Front Med (Lausanne) 4:105
Yan C, Fukushi H, Matsudate H, Ishihara K, Yasuda K, Kitagawa H, Yamaguchi T, Hirai K (2000) Seroepidemiological investigation of feline chlamydiosis in cats and humans in Japan. Microbiol Immunol 44:155–160
Yin L, Kalmar ID, Boden J, Vanrompay D (2015) Chlamydia psittaci infections in Chinese poultry: a literature review. Worlds Poult Sci J 71:473–482
Zhang Y, Cui P, Zhang HC, Wu HL, Ye MZ, Zhu YM, Ai JW, Zhang WH (2020) Clinical application and evaluation of metagenomic next-generation sequencing in suspected adult central nervous system infection. J Transl Med 18:199
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry
Borel, N., Sachse, K. (2023). Zoonotic Transmission of Chlamydia spp.: Known for 140 Years, but Still Underestimated. In: Sing, A. (eds) Zoonoses: Infections Affecting Humans and Animals. Springer, Cham. https://doi.org/10.1007/978-3-030-85877-3_53-1
Download citation
DOI: https://doi.org/10.1007/978-3-030-85877-3_53-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-85877-3
Online ISBN: 978-3-030-85877-3
eBook Packages: Springer Reference MedicineReference Module Medicine