Abstract
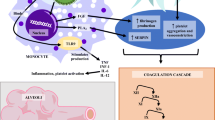
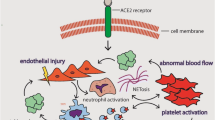
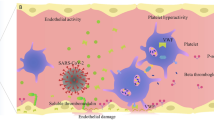
Thrombin is the cornerstone of the coagulation process. Without thrombin, fibrinogen cannot be converted into fibrin, and no stable clot can be formed. Thrombin generation (TG) is a complex process; however, the major driver of in vivo thrombin generation is tissue factor (TF). TF is in turn produced when the endothelial layer is destroyed (finalistic process) but can be released by a number of cells, and namely monocytes (blood-borne TF). In this second case, under inflammatory/infective reaction, TF represents the link between inflammation and coagulation. Given the infective and inflammatory pattern of COVID-19, the pattern of TG in severe cases of COVID-19 is of potential interest, particularly in the setting of the well-known thrombotic complications of this disease. TG can be addressed with different laboratory assays; however, the conventional tests (prothrombin time and activated partial thromboplastin time) are highly nonspecific, and offer a limited information. Point-of-care coagulation tests reflect similar limitations when the reaction time is considered as a marker of TG. Therefore, more specific, nonconventional tests are required to address TG in the setting of COVID-19. These include computerized automated thrombography (CAT), the measure of thrombin inactivation by antithrombin (thrombin-antithrombin complex, TAT), and of the marker of TG prothrombin fragment 1.2 (PF 1.2). Even considering that many biases are to be considered (severity of the disease, presence of thromboembolic complications, and, most of all, different degrees of pharmacological anticoagulation), the existing studies offer a scenario of a “thrombin burst” that is probably of higher degree than what is observed in other sepsis patterns, and that often appears resistant to the standard anticoagulant therapies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62:699–707.
Morrissey JH. Tissue factor: a key molecule in hemostatic and nonhemostatic systems. Int J Hematol. 2004;79:103–8.
Mann KG. Thrombin formation. Chest. 2003;124(Suppl 3):4S–10S.
Dahlback B. Progress in the understanding of the protein C anticoagulant pathway. Int J Hematol. 2004;79:109–16.
Toukh M, Siemens DR, Black A, et al. Thromboelastography identifies hypercoagulablilty and predicts thromboembolic complications in patients with prostate cancer. Thromb Res. 2014;133:88–95.
Liang H, Yang CX, Li H, Wen XJ, Zhou QL, Gu MN. The effects of preloading infusion with hydroxyethyl starch 200/0.5 or 130/0.4 solution on hypercoagulability and excessive platelet activation of patients with colon cancer. Blood Coagul Fibrinolysis. 2010;21:406–13.
Wang Z-W, Ye P-J. Clinical analysis of acute cerebral infarction accompanied with lung cancer. J Acute Dis. 2016;5:307.
Butler MJ. Thromboelastography during and after elective abdominal surgery. Thromb Haemost. 1978;39:488–95.
Howland WS, Castro EB, Fortner JB, Gould P. Proceedings: hypercoagulability. Thromboelastographic monitoring during extensive hepatic surgery. Arch Surg. 1974;108:605–8.
Schmidt DE, Chaireti R, Bruzelius M, Holmström M, Antovic J, Ågren A. Correlation of thromboelastography and thrombin generation assays in warfarin-treated patients. Thromb Res. 2019;178:34–40.
Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C. Use of calibrated automated thrombinography +/− thrombomodulin to recognize the prothrombotic phenotype. Thromb Haemost. 2006;96:562–7.
Tripodi A. The long-awaited whole-blood thrombin generation test. Clin Chem. 2012;58:1173–5.
White D, MacDonald S, Edwards T, et al. Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int J Lab Hematol. 2021;43:123–30.
Nougier C, Benoit R, Dimon M, et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost. 2020;18:2215–9.
Chistolini A, Ruberto F, Alessandri F, et al. Effect of low or high doses of low-molecular-weight heparin on thrombin generation and other haemostasis parameters in critically ill patients with COVID-19. Br J Haematol. 2020;190:e214–8.
Bouck EG, Denorme F, Holle LA, et al. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol. 2021;41:401–14.
Innocenti F, Gori AM, Giusti B, et al. Prognostic value of sepsis-induced coagulation abnormalities: an early assessment in the emergency department. Intern Emerg Med. 2019;14:459–66.
Koyama K, Madoiwa S, Nunomiya S, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care. 2014;18:R13.
Leithäuser B, Matthias FR, Nicolai U, Voss R. Hemostatic abnormalities and the severity of illness in patients at the onset of clinically defined sepsis. Possible indication of the degree of endothelial cell activation? Intensive Care Med. 1996;22:631–6.
Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869–78.
Moosavi M, Wooten M, Goodman A, et al. Retrospective analyses associate hemostasis activation biomarkers with poor outcomes in patients with COVID-19. Am J Clin Pathol. 2021;155:498–505.
Blasi A, von Meijenfeldt FA, Adelmeijer J, et al. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J Thromb Haemost. 2020;18:2646–53.
Jin X, Duan Y, Bao T, et al. The values of coagulation function in COVID-19 patients. PLoS One. 2020;15:e0241329.
Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs. 2007;67:1429–40.
Pleym H, Videm V, Wahba A, et al. Heparin resistance and increased platelet activation in coronary surgery patients treated with enoxaparin preoperatively. Eur J Cardiothorac Surg. 2006;29:933–40.
Ranucci M, Isgrò G, Cazzaniga A, Soro G, Menicanti L, Frigiola A. Predictors for heparin resistance in patients undergoing coronary artery bypass grafting. Perfusion. 1999;14:437–42.
Ranucci M, Antithrombin III. Key factor in extracorporeal circulation. Minerva Anestesiol. 2002;68:454–7.
Dujardin RWG, Hilderink BN, Haksteen WE, et al. Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients. Thromb Res. 2020;196:308–12.
Calderon-Lopez MT, Garcia-Leon N, Gomez-Arevalillo S, Martin-Serrano P, Matilla-Garcia A. Coronavirus disease 2019 and coagulopathy: other prothrombotic coagulation factors. Blood Coagul Fibrinolysis. 2021;32:44–9.
Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–51.
Corrêa TD, Cordioli RL, Campos Guerra JC, et al. Coagulation profile of COVID-19 patients admitted to the ICU: an exploratory study. PLoS One. 2020;15:e0243604.
Zhang Y, Cao W, Jiang W, et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50:580–6.
Gazzaruso C, Paolozzi E, Valenti C, et al. Association between antithrombin and mortality in patients with COVID-19. A possible link with obesity. Nutr Metab Cardiovasc Dis. 2020;30:1914–9.
Lippi G, Henry BM, Sanchis-Gomar F. Plasma antithrombin values are significantly decreased in Coronavirus Disease 2019 (COVID-19) patients with severe illness. Semin Thromb Hemost. 2020; https://doi.org/10.1055/s-0040-1716873.
Capecchi M, Scalambrino E, Griffini S, et al. Relationship between thrombin generation parameters and prothrombin fragment 1 + 2 plasma levels. Int J Lab Hematol. 2021; https://doi.org/10.1111/ijlh.13462.
Koskela SM, Joutsi-Korhonen L, Mäkelä SM, et al. Diminished coagulation capacity assessed by calibrated automated thrombography during acute Puumala hantavirus infection. Blood Coagul Fibrinolysis. 2018;29:55–60.
Joly B, Barbay V, Borg JY, Le Cam-Duchez V. Comparison of markers of coagulation activation and thrombin generation test in uncomplicated pregnancies. Thromb Res. 2013;132:386–91.
Nahab F, Sharashidze V, Liu M, et al. Markers of coagulation and hemostatic activation aid in identifying causes of cryptogenic stroke. Neurology. 2020;94:e1892–9.
Liu M, Ellis D, Duncan A, Belagaje S, Belair T, Henriquez L, Rangaraju S, Nahab F. The utility of the markers of coagulation and hemostatic activation profile in the management of embolic strokes of undetermined source. J Stroke Cerebrovasc Dis. 2021;30:105592.
Skjeflo EW, Christiansen D, Fure H, et al. Staphylococcus aureus-induced complement activation promotes tissue factor-mediated coagulation. J Thromb Haemost. 2018;16:905–18.
Okamoto K, Takaki A, Takeda S, Katoh H, Ohsato K. Coagulopathy in disseminated intravascular coagulation due to abdominal sepsis: determination of prothrombin fragment 1 + 2 and other markers. Haemostasis. 1992;22:17–24.
Hoppensteadt D, Tsuruta K, Cunanan J, et al. Thrombin generation mediators and markers in sepsis-associated coagulopathy and their modulation by recombinant thrombomodulin. Clin Appl Thromb Hemost. 2014;20:129–35.
Prakash S, Verghese S, Roxby D, Dixon D, Bihari S, Bersten A. Changes in fibrinolysis and severity of organ failure in sepsis: a prospective observational study using point-of-care test--ROTEM. J Crit Care. 2015;30:264–70.
Al-Samkari H, Song F, Van Cott EM, Kuter DJ, Rosovsky R. Evaluation of the prothrombin fragment 1.2 in patients with coronavirus disease 2019 (COVID-19). Am J Hematol. 2020;95:1479–85.
Ranucci M, Sitzia C, Baryshnikova E, et al. Covid-19-Associated coagulopathy: biomarkers of thrombin generation and fibrinolysis leading the outcome. J Clin Med. 2020;9:3487.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ranucci, M., Aloisio, T. (2022). COVID-19 Associated Coagulopathy: The Thrombin Burst. In: Ranucci, M. (eds) The Coagulation Labyrinth of Covid-19. Springer, Cham. https://doi.org/10.1007/978-3-030-82938-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-82938-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82937-7
Online ISBN: 978-3-030-82938-4
eBook Packages: MedicineMedicine (R0)