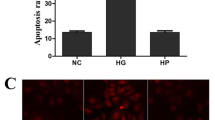
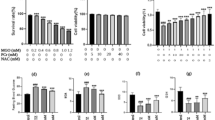
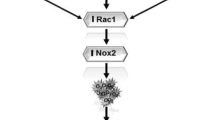
Abstract
Increased oxidative stress has been recognized as a major contributing factor to various pathological conditions including diabetes and its complications. Although, several mechanisms contribute to increased oxidative stress (OxS) in diabetes, increased levels of glucose and altered metabolic activities are major contributors to production of reactive oxygen species (ROS) and OxS. The identity of the target cells and metabolic pathways involved in ROS production provide a venue for treatment of the underlying causes and mitigation of diabetes complications including diabetic retinopathy. Diabetic retinopathy affects retinal neurovasculature, and loss of retinal vascular pericytes (PC) has been recognized as one of the early targets. We have shown that retinal PC are most sensitive to high glucose conditions in culture compared with retinal vascular endothelial cells (EC) and astrocytes (AC). We have proposed that retinal PC may differ in their metabolism of glucose compared with EC. Pericytes likely prefer oxidative metabolism for energy production, especially under high glucose conditions, generating excess ROS that drives their demise. This is mediated, in part, through activation of hexose biosynthetic pathway, enhanced O-GlcNAc modification and stabilization of P53, and attenuation of the Warburg effect. In support of this hypothesis, we recently showed retinal PC, but not EC, generate more superoxide under high glucose conditions. We also showed inhibition of carbonic anhydrases (CA) protects PC from adverse effects of high glucose. The inhibition of CA, especially those in the mitochondria (mCA), limits the production of bicarbonate that is essential for the first step of oxidative metabolism in the mitochondria. Thus, targeting of the mCA may provide a unique opportunity for modulation of PC metabolism mitigating the development and progression of diabetic retinopathy and likely other complications of diabetes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AC:
-
Astrocytes
- AGE:
-
Advanced glycation end product
- AGER:
-
AGE receptor
- CA:
-
Carbonic anhydrases
- EC:
-
Endothelial cells
- mCA:
-
Mitochondrial CA
- OxS:
-
Oxidative stress
- PC:
-
Pericytes
- RPE:
-
Retinal pigment epithelium
- ROS:
-
Reactive oxygen species
- SMC:
-
Smooth muscle cells
- STZ:
-
Streptozotocin
- 8OHdG:
-
8-Hydroxy-2’-deoxyguanosine
References
Aboualizadeh E, Ranji M, Sorenson CM, Sepehr R, Sheibani N, Hirschmugl CJ (2017) Retinal oxidative stress at the onset of diabetes determined by synchrotron FTIR widefield imaging: towards diabetes pathogenesis. Analyst 142(7):1061–1072. https://doi.org/10.1039/c6an02603f
Agardh CD, Agardh E, Torffvit O (1997) The association between retinopathy, nephropathy, cardiovascular disease and long-term metabolic control in type 1 diabetes mellitus: a 5 year follow-up study of 442 adult patients in routine care. Diabetes Res Clin Pract 35(2–3):113
Aghdam SY, Sheibani N (2013) The ubiquitin-proteasome system and microvascular complications of diabetes. J Ophthalmic vis Res 8(3):244–256
Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE (1995) Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A 92(23):10457–10461
Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW (2012) Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (silver Spring) 20(2):330–342. https://doi.org/10.1038/oby.2011.330
Ansari NH, Zhang W, Fulep E, Mansour A (1998) Prevention of pericyte loss by trolox in diabetic rat retina. J Toxicol Environ Health A 54(6):467–475
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366(13):1227–1239. https://doi.org/10.1056/NEJMra1005073
Arechederra RL, Waheed A, Sly WS, Supuran CT, Minteer SD (2013) Effect of sulfonamides as carbonic anhydrase VA and VB inhibitors on mitochondrial metabolic energy conversion. Bioorg Med Chem 21(6):1544–1548. https://doi.org/10.1016/j.bmc.2012.06.053
Bae ON, Wang JM, Baek SH, Wang Q, Yuan H, Chen AF (2013) Oxidative stress-mediated thrombospondin-2 upregulation impairs bone marrow-derived angiogenic cell function in diabetes mellitus. Arterioscler Thromb Vasc Biol 33(8):1920–1927. https://doi.org/10.1161/atvbaha.113.301609
Bahtiyar G, Gutterman D, Lebovitz H (2016) Heart failure: a major cardiovascular complication of diabetes mellitus. Curr Diab Rep 16(11):116. https://doi.org/10.1007/s11892-016-0809-4
Balasubramanyam M, Premanand C, Mohan V (2002) The lymphocyte as a cellular model to study insights into the pathophysiology of diabetes and its complications. Ann N Y Acad Sci 958:399–402
Ben-Menachem E (1996) Expanding antiepileptic drug options: clinical efficacy of new therapeutic agents. Epilepsia 37(Suppl 2):S4–S7
Bettaieb A, Bakke J, Nagata N, Matsuo K, Xi Y, Liu S, AbouBechara D, Melhem R, Stanhope K, Cummings B, Graham J, Bremer A, Zhang S, Lyssiotis CA, Zhang ZY, Cantley LC, Havel PJ, Haj FG (2013) Protein tyrosine phosphatase 1B regulates pyruvate kinase M2 tyrosine phosphorylation. J Biol Chem 288(24):17360–17371. https://doi.org/10.1074/jbc.M112.441469
Betz AL, Bowman PD, Goldstein GW (1983) Hexose transport in microvascular endothelial cells cultured from bovine retina. Exp Eye Res 36(2):269–277
Bhavsar AR (2006) Diabetic retinopathy: the latest in current management. Retina 26(6 Suppl):S71-79. https://doi.org/10.1097/01.iae.0000236466.23640.c9
Boddy A, Edwards P, Rowland M (1989) Binding of sulfonamides to carbonic anhydrase: influence on distribution within blood and on pharmacokinetics. Pharm Res 6(3):203–209
Boeri D, Maiello M, Lorenzi M (2001) Increased prevalence of microthromboses in retinal capillaries of diabetic individuals. Diabetes 50(6):1432–1439
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865):813–820
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625
Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Caldwell RW (2003) Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 19(6):442–455. https://doi.org/10.1002/dmrr.415[doi]
Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW (2005) Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets 6(4):511–524
Campesi I, Franconi F, Seghieri G, Meloni M (2017) Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol Res 119:195–207. https://doi.org/10.1016/j.phrs.2017.01.023
Carter ND, Dodgson SJ, Quant PA (1990) Expression of hepatic mitochondrial carbonic anhydrase V. Biochim Biophys Acta 1036(3):237–241
Chan PS, Kanwar M, Kowluru RA (2010) Resistance of retinal inflammatory mediators to suppress after reinstitution of good glycemic control: novel mechanism for metabolic memory. J Diabetes Complications 24(1):55–63. https://doi.org/10.1016/j.jdiacomp.2008.10.002
Chappell JB, Crofts AR (1965) Calcium ion accumulation and volume changes of isolated liver mitochondria. Calcium Ion-Induced Swelling Biochem J 95:378–386. https://doi.org/10.1042/bj0950378
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278(38):36027–36031. https://doi.org/10.1074/jbc.M304854200
Chen P, Guo AM, Edwards PA, Trick G, Scicli AG (2007) Role of NADPH oxidase and ANG II in diabetes-induced retinal leukostasis. Am J Physiol Regul Integr Comp Physiol 293(4):R1619-1629. https://doi.org/10.1152/ajpregu.00290.2007
Cogan DG, Toussaint D, Kuwabara T (1961) Retinal vascular patterns. IV. Diabetic Retinopathy. Arch Ophthalmol 66:366–378
Cogan DG, Kinoshita JH, Kador PF, Robison G, Datilis MB, Cobo LM, Kupfer C (1984) NIH conference. Aldose reductase and complications of diabetes. Ann Intern Med 101 (1):82–91
Cohen PP (1981) The ornithine-urea cycle: biosynthesis and regulation of carbamyl phosphate synthetase I and ornithine transcarbamylase. Curr Top Cell Regul 18:1–19
Corcoran SE, O’Neill LA (2016) HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest 126(10):3699–3707. https://doi.org/10.1172/jci84431
Costa PZ, Soares R (2013) Neovascularization in diabetes and its complications. Unraveling the Angiogenic Paradox Life Sci 92(22):1037–1045. https://doi.org/10.1016/j.lfs.2013.04.001
Dagher Z, Ruderman N, Tornheim K, Ido Y (2001) Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ Res 88(12):1276–1282
Das Evcimen N, Ulusu NN, Karasu C, Dogru B (2004) Adenosine triphosphatase activity of streptozotocin-induced diabetic rat brain microsomes. Effect of vitamin E. Gen Physiol Biophys 23(3):347–355
De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154(3):651–663. https://doi.org/10.1016/j.cell.2013.06.037
De Simone G, Di Fiore A, Menchise V, Pedone C, Antel J, Casini A, Scozzafava A, Wurl M, Supuran CT (2005) Carbonic anhydrase inhibitors. Zonisamide is an effective inhibitor of the cytosolic isozyme II and mitochondrial isozyme V: solution and X-ray crystallographic studies. Bioorg Med Chem Lett 15(9):2315–2320. https://doi.org/10.1016/j.bmcl.2005.03.032
Deutsch SI, Schwartz BL, Rosse RB, Mastropaolo J, Marvel CL, Drapalski AL (2003) Adjuvant topiramate administration: a pharmacologic strategy for addressing NMDA receptor hypofunction in schizophrenia. Clin Neuropharmacol 26(4):199–206
Devi TS, Hosoya K, Terasaki T, Singh LP (2013) Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: implications for diabetic retinopathy. Exp Cell Res 319(7):1001–1012. https://doi.org/10.1016/j.yexcr.2013.01.012
Ding Y, Sun X, Shan PF (2017) MicroRNAs and cardiovascular disease in diabetes mellitus. Biomed Res Int 2017:4080364. https://doi.org/10.1155/2017/4080364
Dodgson SJ, Contino LC (1988) Rat kidney mitochondrial carbonic anhydrase. Arch Biochem Biophys 260(1):334–341
Dodgson SJ, Forster RE (1986) Carbonic anhydrase: inhibition results in decreased urea production by hepatocytes. J Appl Physiol (Bethesda, Md: 1985) 60(2):646–652. https://doi.org/10.1152/jappl.1986.60.2.646
Dodgson SJ, Forster RE 2nd (1986b) Inhibition of CA V decreases glucose synthesis from pyruvate. Arch Biochem Biophys 251(1):198–204
Dodgson SJ, Forster RE 2nd, Storey BT, Mela L (1980) Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A 77(9):5562–5566
Dodgson SJ, Forster RE 2nd, Schwed DA, Storey BT (1983) Contribution of matrix carbonic anhydrase to citrulline synthesis in isolated guinea pig liver mitochondria. J Biol Chem 258(12):7696–7701
Dodgson SJ, Shank RP, Maryanoff BE (2000) Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia 41(Suppl 1):S35-39
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. https://doi.org/10.1152/physrev.00018.2001
Duarte DA, Silva KC, Rosales MA, Lopes de Faria JB, Lopes de Faria JM (2013) The concomitance of hypertension and diabetes exacerbating retinopathy: the role of inflammation and oxidative stress. Curr Clin Pharmacol 8(4):266–277
Dugan LL, You Y-H, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves A-E, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K (2013) AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Investig 123(11):4888–4899. https://doi.org/10.1172/JCI66218
Duh EJ, Sun JK, Stitt AW (2017) Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI insight 2 (14). https://doi.org/10.1172/jci.insight.93751
Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 97(22):12222–12226. https://doi.org/10.1073/pnas.97.22.12222
Du Y, Smith MA, Miller CM, Kern TS (2002) Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem 80(5):771–779
Du Y, Miller CM, Kern TS (2003) Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 35(11):1491–1499
Du Y, Veenstra A, Palczewski K, Kern TS (2013) Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A 110(41):16586–16591. https://doi.org/10.1073/pnas.1314575110
Eelen G, Cruys B, Welti J, De Bock K, Carmeliet P (2013) Control of vessel sprouting by genetic and metabolic determinants. Trends Endocrinol Metab 24(12):589–596. https://doi.org/10.1016/j.tem.2013.08.006
Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW (2008) Importance of pericytes and mechanisms of pericyte loss during diabetic retinopathy. Diabetes Obes Metab 10(1):53–63. https://doi.org/10.1111/j.1463-1326.2007.00795.x
Favret S, Binet F, Lapalme E, Leboeuf D, Carbadillo J, Rubic T, Picard E, Mawambo G, Tetreault N, Joyal JS, Chemtob S, Sennlaub F, Sangiovanni JP, Guimond M, Sapieha P (2013) Deficiency in the metabolite receptor SUCNR1 (GPR91) leads to outer retinal lesions. Aging (albany NY) 5(6):427–444
Frey T, Antonetti DA (2011) Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal 15(5):1271–1284. https://doi.org/10.1089/ars.2011.3906
Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S (1999) Human mitochondrial carbonic anhydrase VB. cDNA cloning, mRNA expression, subcellular localization, and mapping to chromosome x. J Biol Chem 274(30):21228–21233. https://doi.org/10.1074/jbc.274.30.21228
Gadde KM, Franciscy DM, Wagner HR 2nd, Krishnan KR (2003) Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA 289(14):1820–1825. https://doi.org/10.1001/jama.289.14.1820
Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW (2011) Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 377(9774):1341–1352. https://doi.org/10.1016/S0140-6736(11)60205-5
Gelisken O, Gelisken F, Ozcetin H (1990) Treatment of chronic macular oedema with low dosage acetazolamide. Bull Soc Belge Ophtalmol 238:153–160
Ghanian Z, Mehrvar S, Jamali N, Sheibani N, Ranji M (2018) Time-lapse microscopy of oxidative stress demonstrates metabolic sensitivity of retinal pericytes under high glucose condition. J Biophotonics 11(9):e201700289. https://doi.org/10.1002/jbio.201700289
Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P (2014) Metabolism of stromal and immune cells in health and disease. Nature 511(7508):167–176. https://doi.org/10.1038/nature13312
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070. https://doi.org/10.1161/circresaha.110.223545
Groschner LN, Waldeck-Weiermair M, Malli R, Graier WF (2012) Endothelial mitochondria–less respiration, more integration. Pflugers Arch 464(1):63–76. https://doi.org/10.1007/s00424-012-1085-z
Groussard C, Morel I, Chevanne M, Monnier M, Cillard J, Delamarche A (2000) Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. Journal of applied physiology (Bethesda, Md: 1985) 89(1):169–175. https://doi.org/10.1152/jappl.2000.89.1.169
Gurcel C, Vercoutter-Edouart AS, Fonbonne C, Mortuaire M, Salvador A, Michalski JC, Lemoine J (2008) Identification of new O-GlcNAc modified proteins using a click-chemistry-based tagging. Anal Bioanal Chem 390(8):2089–2097. https://doi.org/10.1007/s00216-008-1950-y
Gurel Z, Sieg KM, Shallow KD, Sorenson CM, Sheibani N (2013) Retinal O-linked N-acetylglucosamine protein modifications: implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Mol vis 19:1047–1059
Gurel Z, Zaro BW, Pratt MR, Sheibani N (2014) Identification of O-GlcNAc modification targets in mouse retinal pericytes: implication of p53 in pathogenesis of diabetic retinopathy. PLoS ONE 9(5):e95561. https://doi.org/10.1371/journal.pone.0095561
Guzel O, Innocenti A, Scozzafava A, Salman A, Supuran CT (2009) Carbonic anhydrase inhibitors. Aromatic/heterocyclic sulfonamides incorporating phenacetyl, pyridylacetyl and thienylacetyl tails act as potent inhibitors of human mitochondrial isoforms VA and VB. Bioorg Med Chem 17(14):4894–4899. https://doi.org/10.1016/j.bmc.2009.06.006
Haefliger IO, Zschauer A, Anderson DR (1994) Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Invest Ophthalmol vis Sci 35(3):991–997
Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M (1991) Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Natl Acad Sci U S A 88(24):11555–11558
Hammes HP, Bartmann A, Engel L, Wulfroth P (1997) Antioxidant treatment of experimental diabetic retinopathy in rats with nicanartine. Diabetologia 40(6):629–634. https://doi.org/10.1007/s001250050726
Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U (2002) Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51(10):3107–3112
Hammes HP, Feng Y, Pfister F, Brownlee M (2011) Diabetic retinopathy: targeting vasoregression. Diabetes 60(1):9–16. https://doi.org/10.2337/db10-0454
Hayden MR, Yang Y, Habibi J, Bagree SV, Sowers JR (2010) Pericytopathy: Oxidative stress and impaired cellular longevity in the pancreas and skeletal muscle in metabolic syndrome and type 2 diabetes. Oxid Med Cell Longev 3(5):290–303
Hazen SA, Waheed A, Sly WS, LaNoue KF, Lynch CJ (1996) Differentiation-dependent expression of CA V and the role of carbonic anhydrase isozymes in pyruvate carboxylation in adipocytes. FASEB J 10(4):481–490
Hegde KR, Kovtun S, Varma SD (2010) Inhibition of glycolysis in the retina by oxidative stress: prevention by pyruvate. Mol Cell Biochem 343(1–2):101–105. https://doi.org/10.1007/s11010-010-0503-9
Hewett-Emmett D, Tashian RE (1996) Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol Phylogenet Evol 5(1):50–77. https://doi.org/10.1006/mpev.1996.0006
Huang Q, Sheibani N (2008) High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol 295(6):C1647-1657
Ibrahim AS, El-Remessy AB, Matragoon S, Zhang W, Patel Y, Khan S, Al-Gayyar MM, El-Shishtawy MM, Liou GI (2011) Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes 60(4):1122–1133. https://doi.org/10.2337/db10-1160
Jha JC, Ho F, Dan C, Jandeleit-Dahm K (2018) A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci (lond) 132(16):1811–1836. https://doi.org/10.1042/CS20171459
Jitrapakdee S, St Maurice M, Rayment I, Cleland WW, Wallace JC, Attwood PV (2008) Structure, mechanism and regulation of pyruvate carboxylase. Biochem J 413(3):369–387. https://doi.org/10.1042/BJ20080709
Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP (2004) A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 18(12):1450–1452. https://doi.org/10.1096/fj.03-1476fje
Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL (1993) Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 42(1):80–89
Karler R, Woodbury DM (1960) Intracellular distribution of carbonic anhydrase. Biochem J 75:538–543. https://doi.org/10.1042/bj0750538
Kaur H, Halliwell B (1994) Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett 350(1):9–12
Kellett MW, Smith DF, Stockton PA, Chadwick DW (1999) Topiramate in clinical practice: first year’s postlicensing experience in a specialist epilepsy clinic. J Neurol Neurosurg Psychiatry 66(6):759–763. https://doi.org/10.1136/jnnp.66.6.759
Kim YH, Kim YS, Roh GS, Choi WS, Cho GJ (2012) Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol 90(1):e31-37. https://doi.org/10.1111/j.1755-3768.2011.02243.x
King GL (2008) The role of inflammatory cytokines in diabetes and its complications. J Periodontol 79(8 Suppl):1527–1534. https://doi.org/10.1902/jop.2008.080246
Kowluru RA, Abbas SN (2003) Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol vis Sci 44(12):5327–5334. https://doi.org/10.1167/iovs.03-0353
Kowluru RA, Odenbach S (2004a) Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol 88(10):1343–1347. https://doi.org/10.1136/bjo.2003.038133
Kowluru RA, Odenbach S (2004b) Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol vis Sci 45:4161–4166. https://doi.org/10.1167/iovs.04-0633
Kowluru RA, Atasi L, Ho YS (2006) Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol vis Sci 47(4):1594–1599. https://doi.org/10.1167/iovs.05-1276
Kowluru RA, Zhong Q, Kanwar M (2010) Metabolic memory and diabetic retinopathy: role of inflammatory mediators in retinal pericytes. Exp Eye Res 90(5):617–623. https://doi.org/10.1016/j.exer.2010.02.006
Koya D, King GL (1998) Protein kinase C activation and the development of diabetic complications. Diabetes 47(6):859–866
Kubo E, Singh DP, Fatma N, Akagi Y (2009) TAT-mediated peroxiredoxin 5 and 6 protein transduction protects against high-glucose-induced cytotoxicity in retinal pericytes. Life Sci 84(23–24):857–864. https://doi.org/10.1016/j.lfs.2009.03.019
Kuwabara T, Carroll JM, Cogan DG (1961) Retinal vascular patterns. III. Age, hypertension, absolute glaucoma, injury. Arch Ophthalmol 65:708–716
LeDoux SP, Driggers WJ, Hollensworth BS, Wilson GL (1999) Repair of alkylation and oxidative damage in mitochondrial DNA. Mutat Res 434(3):149–159
Leniger T, Thone J, Wiemann M (2004) Topiramate modulates pH of hippocampal CA3 neurons by combined effects on carbonic anhydrase and Cl-/HCO3-exchange. Br J Pharmacol 142(5):831–842. https://doi.org/10.1038/sj.bjp.0705850
Liang Y, Chen X, Osborne M, DeCarlo SO, Jetton TL, Demarest K (2005) Topiramate ameliorates hyperglycaemia and improves glucose-stimulated insulin release in ZDF rats and db/db mice. Diabetes Obes Metab 7(4):360–369. https://doi.org/10.1111/j.1463-1326.2004.00403.x
Lincet H, Icard P (2015) How do glycolytic enzymes favour cancer cell proliferation by nonmetabolic functions? Oncogene 34(29):3751–3759. https://doi.org/10.1038/onc.2014.320
Liu Y, Fiskum G, Schubert D (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80(5):780–787
Li W, Chan LS, Khatami M, Rockey JH (1985) Characterization of glucose transport by bovine retinal capillary pericytes in culture. Exp Eye Res 41(2):191–199
Li W, Yanoff M, Jian B, He Z (1999) Altered mRNA levels of antioxidant enzymes in pre-apoptotic pericytes from human diabetic retinas. Cell Mol Biol (Noisy-le-grand) 45(1):59–66
Li T, Hu J, Du S, Chen Y, Wang S, Wu Q (2014) ERK1/2/COX-2/PGE2 signaling pathway mediates GPR91-dependent VEGF release in streptozotocin-induced diabetes. Mol vis 20:1109–1121
Lusty CJ (1978) Carbamyl phosphate synthetase. Bicarbonate-dependent hydrolysis of ATP and potassium activation. J Biol Chem 253(12):4270–4278
Lynch CJ, Fox H, Hazen SA, Stanley BA, Dodgson S, Lanoue KF (1995) Role of hepatic carbonic anhydrase in de novo lipogenesis. Biochem J 310(Pt 1):197–202. https://doi.org/10.1042/bj3100197
Mandarino LJ, Finlayson J, Hassell JR (1994) High glucose downregulates glucose transport activity in retinal capillary pericytes but not endothelial cells. Invest Ophthalmol vis Sci 35(3):964–972
Maresca A, Supuran CT (2011) (R)-/(S)-10-camphorsulfonyl-substituted aromatic/heterocyclic sulfonamides selectively inhibit mitochondrial over cytosolic carbonic anhydrases. Bioorg Med Chem Lett 21(5):1334–1337. https://doi.org/10.1016/j.bmcl.2011.01.050
Mazurek S (2011) Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43(7):969–980. https://doi.org/10.1016/j.biocel.2010.02.005
Mertens S, Noll T, Spahr R, Krutzfeldt A, Piper HM (1990) Energetic response of coronary endothelial cells to hypoxia. Am J Physiol 258(3 Pt 2):H689-694
Miller AG, Smith DG, Bhat M, Nagaraj RH (2006) Glyoxalase I is critical for human retinal capillary pericyte survival under hyperglycemic conditions. J Biol Chem 281(17):11864–11871. https://doi.org/10.1074/jbc.M513813200
Mizutani M, Kern TS, Lorenzi M (1996) Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Investig 97(12):2883–2890. https://doi.org/10.1172/jci118746
Mohamed IN, Soliman SA, Alhusban A, Matragoon S, Pillai BA, Elmarkaby AA, El-Remessy AB (2012) Diabetes exacerbates retinal oxidative stress, inflammation, and microvascular degeneration in spontaneously hypertensive rats. Mol vis 18:1457–1466
Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Münch G, Wood AG, Forbes J, Greenaway TM, Pearson S, Srikanth V (2013) Brain atrophy in type 2 diabetes. Reg Distrib Influence Cognition 36(12):4036–4042. https://doi.org/10.2337/dc13-0143
Mori K, Ogawa Y, Ebihara K, Tamura N, Tashiro K, Kuwahara T, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, Nakao K (1999) Isolation and characterization of CA XIV, a novel membrane-bound carbonic anhydrase from mouse kidney. J Biol Chem 274(22):15701–15705. https://doi.org/10.1074/jbc.274.22.15701
Morohoshi K, Ohbayashi M, Patel N, Chong V, Bird AC, Ono SJ (2012) Identification of anti-retinal antibodies in patients with age-related macular degeneration. Exp Mol Pathol 93(2):193–199. https://doi.org/10.1016/j.yexmp.2012.03.007
Nagao Y, Platero JS, Waheed A, Sly WS (1993) Human mitochondrial carbonic anhydrase: cDNA cloning, expression, subcellular localization, and mapping to chromosome 16. Proc Natl Acad Sci U S A 90(16):7623–7627. https://doi.org/10.1073/pnas.90.16.7623
Nagao Y, Batanian JR, Clemente MF, Sly WS (1995) Genomic organization of the human gene (CA5) and pseudogene for mitochondrial carbonic anhydrase V and their localization to chromosomes 16q and 16p. Genomics 28(3):477–484
Nakamura S, Makita Z, Ishikawa S, Yasumura K, Fujii W, Yanagisawa K, Kawata T, Koike T (1997) Progression of nephropathy in spontaneous diabetic rats is prevented by OPB-9195, a novel inhibitor of advanced glycation. Diabetes 46(5):895–899
Nerlich AG, Sauer U, Kolm-Litty V, Wagner E, Koch M, Schleicher ED (1998) Expression of glutamine:fructose-6-phosphate amidotransferase in human tissues: evidence for high variability and distinct regulation in diabetes. Diabetes 47(2):170–178
Nguyen DV, Shaw LC, Grant MB (2012) Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol (lausanne) 3:170. https://doi.org/10.3389/fendo.2012.00170
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6779):787–790. https://doi.org/10.1038/35008121
Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT (2005) Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. J Med Chem 48(24):7860–7866. doi:https://doi.org/10.1021/jm050483n
Nishimura C, Hotta Y, Gui T, Seko A, Fujimaki T, Ishikawa T, Hayakawa M, Kanai A, Saito T (1997) The level of erythrocyte aldose reductase is associated with the severity of diabetic retinopathy. Diabetes Res Clin Pract 37(3):173–177
Panzhinskiy E, Ren J, Nair S (2013) Pharmacological inhibition of protein tyrosine phosphatase 1B: a promising strategy for the treatment of obesity and type 2 diabetes mellitus. Curr Med Chem 20(21):2609–2625
Patrick P, Price TO, Diogo AL, Sheibani N, Banks WA, Shah GN (2015) Topiramate protects pericytes from glucotoxicity: role for mitochondrial CA VA in cerebromicrovascular disease in diabetes. J Endocrinol Diabetes 2(2)
Picard F, Deshaies Y, Lalonde J, Samson P, Richard D (2000) Topiramate reduces energy and fat gains in lean (Fa/?) and obese (fa/fa) Zucker rats. Obes Res 8(9):656–663. https://doi.org/10.1038/oby.2000.84
Podesta F, Romeo G, Liu WH, Krajewski S, Reed JC, Gerhardinger C, Lorenzi M (2000) Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am J Pathol 156(3):1025–1032
Poulsen SA, Wilkinson BL, Innocenti A, Vullo D, Supuran CT (2008) Inhibition of human mitochondrial carbonic anhydrases VA and VB with para-(4-phenyltriazole-1-yl)-benzenesulfonamide derivatives. Bioorg Med Chem Lett 18(16):4624–4627. https://doi.org/10.1016/j.bmcl.2008.07.010
Price TO, Sheibani N (1863) Shah GN (2017) Regulation of high glucose-induced apoptosis of brain pericytes by mitochondrial CA VA: A specific target for prevention of diabetic cerebrovascular pathology. Biochim Biophys Acta Mol Basis Dis 4:929–935. https://doi.org/10.1016/j.bbadis.2017.01.025
Price TO, Eranki V, Banks WA, Ercal N, Shah GN (2012) Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology 153(1):362–372. https://doi.org/10.1210/en.2011-1638
Price TO, Farr SA, Niehoff ML, Ercal N, Morley JE, Shah GN (2015) Protective effect of topiramate on hyperglycemia-induced cerebral oxidative stress, pericyte loss and learning behavior in diabetic mice. Int Lib Diabetes Metab 1(1):6–12
Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP (2001) VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol vis Sci 42(10):2408–2413
Rask-Madsen C, King GL (2013) Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 17(1):20–33. https://doi.org/10.1016/j.cmet.2012.11.012
Richter C, Park JW, Ames BN (1988) Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A 85(17):6465–6467
Roy Chengappa KN, Levine J, Rathore D, Parepally H, Atzert R (2001) Long-term effects of topiramate on bipolar mood instability, weight change and glycemic control: a case-series. Eur Psychiatry J Assoc Eur Psychiatrists 16(3):186–190
Roy MS, Janal MN, Crosby J, Donnelly R (2013) Inflammatory biomarkers and progression of diabetic retinopathy in African Americans with type 1 diabetes. Invest Ophthalmol vis Sci 54(8):5471–5480. https://doi.org/10.1167/iovs.13-12212
Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA (1994) Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269(42):26066–26075
Salameh TS, Shah GN, Price TO, Hayden MR, Banks WA (2016) Blood-brain barrier disruption and neurovascular unit dysfunction in diabetic mice: protection with the mitochondrial carbonic anhydrase inhibitor topiramate. J Pharmacol Exp Ther 359(3):452–459. https://doi.org/10.1124/jpet.116.237057
Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honore JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O, Lachapelle P, Di Polo A, Beausejour C, Andelfinger G, Mitchell G, Sennlaub F, Chemtob S (2008) The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med 14(10):1067–1076. https://doi.org/10.1038/nm.1873
Scheef E, Wang S, Sorenson CM, Sheibani N (2005) Isolation and characterization of murine retinal astrocytes. Mol vis 11:613–624
Scheef EA, Sorenson CM, Sheibani N (2009) Attenuation of proliferation and migration of retinal pericytes in the absence of thrombospondin-1. Am J Physiol Cell Physiol 296(4):C724-734. https://doi.org/10.1152/ajpcell.00409.2008
Schleicher ED, Weigert C (2000) Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int Suppl 77:S13-18
Schmidt D, Jacob R, Loiseau P, Deisenhammer E, Klinger D, Despland A, Egli M, Bauer G, Stenzel E, Blankenhorn V (1993) Zonisamide for add-on treatment of refractory partial epilepsy: a European double-blind trial. Epilepsy Res 15(1):67–73
Scott JA, King GL (2004) Oxidative stress and antioxidant treatment in diabetes. Ann N Y Acad Sci 1031:204–213. https://doi.org/10.1196/annals.1331.020
Scozzafava A, Supuran CT, Carta F (2013) Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat 23(6):725–735. https://doi.org/10.1517/13543776.2013.790957
Shah GN, Hewett-Emmett D, Grubb JH, Migas MC, Fleming RE, Waheed A, Sly WS (2000) Mitochondrial carbonic anhydrase CA VB: differences in tissue distribution and pattern of evolution from those of CA VA suggest distinct physiological roles. Proc Natl Acad Sci U S A 97(4):1677–1682
Shah GN, Morofuji Y, Banks WA, Price TO (2013a) High glucose-induced mitochondrial respiration and reactive oxygen species in mouse cerebral pericytes is reversed by pharmacological inhibition of mitochondrial carbonic anhydrases: Implications for cerebral microvascular disease in diabetes. Biochem Biophys Res Commun 440(2):354–358. https://doi.org/10.1016/j.bbrc.2013.09.086
Shah GN, Price TO, Banks WA, Morofuji Y, Kovac A, Ercal N, Sorenson CM, Shin ES, Sheibani N (2013b) Pharmacological inhibition of mitochondrial carbonic anhydrases protects mouse cerebral pericytes from high glucose-induced oxidative stress and apoptosis. J Pharmacol Exp Ther 344(3):637–645. https://doi.org/10.1124/jpet.112.201400
Shah GN, Rubbelke TS, Hendin J, Nguyen H, Waheed A, Shoemaker JD, Sly WS (2013c) Targeted mutagenesis of mitochondrial carbonic anhydrases VA and VB implicates both enzymes in ammonia detoxification and glucose metabolism. Proc Natl Acad Sci U S A 110(18):7423–7428. https://doi.org/10.1073/pnas.1305805110
Sheikpranbabu S, Haribalaganesh R, Gurunathan S (2011) Pigment epithelium-derived factor inhibits advanced glycation end-products-induced cytotoxicity in retinal pericytes. Diabetes Metab 37(6):505–511. https://doi.org/10.1016/j.diabet.2011.03.006
Shin ES, Sorenson CM, Sheibani N (2014a) Diabetes and retinal vascular dysfunction. J Ophthalmic vis Res 9(3):362–373. https://doi.org/10.4103/2008-322x.143378
Shin ES, Huang Q, Gurel Z, Sorenson CM, Sheibani N (2014b) High glucose alters retinal astrocytes phenotype through increased production of inflammatory cytokines and oxidative stress. PLoS ONE 9(7):e103148. https://doi.org/10.1371/journal.pone.0103148
Shin ES, Huang Q, Gurel Z, Palenski TL, Zaitoun I, Sorenson CM, Sheibani N (2014c) STAT1-mediated Bim expression promotes the apoptosis of retinal pericytes under high glucose conditions. Cell Death Dis 5:e986. https://doi.org/10.1038/cddis.2013.517
Singh LP, Devi TS, Yumnamcha T, Devi TS, Somayajulu M, Kowluru RA, Singh LP (2017) The role of Txnip in Mitophagy dysregulation and inflammasome activation in diabetic retinopathy: a new perspective TXNIP regulates mitophagy in retinal Muller cells under high-glucose conditions: implications for diabetic retinopathy. JOJ Ophthalmology 4(4):e2777. https://doi.org/10.19080/jojo.2017.04.555643
Sly WS, Hu PY (1995) Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 64:375–401. https://doi.org/10.1146/annurev.bi.64.070195.002111
Smaine FZ, Pacchiano F, Rami M, Barragan-Montero V, Vullo D, Scozzafava A, Winum JY, Supuran CT (2008) Carbonic anhydrase inhibitors: 2-substituted-1,3,4-thiadiazole-5-sulfamides act as powerful and selective inhibitors of the mitochondrial isozymes VA and VB over the cytosolic and membrane-associated carbonic anhydrases I, II and IV. Bioorg Med Chem Lett 18(24):6332–6335. https://doi.org/10.1016/j.bmcl.2008.10.093
Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW (2017) Diabetic retinopathy: a position statement by the American diabetes association. Diabetes Care 40(3):412–418. https://doi.org/10.2337/dc16-2641
Spatz M, Mrsulja BB, Wroblewska B, Merkel N, Bembry J (1986) Modulation of glycogen metabolism in cerebromicrovascular smooth muscle and endothelial cultures. Biochem Biophys Res Commun 134(2):484–491
Stadtman ER, Levine RL (2000) Protein oxidation. Ann N Y Acad Sci 899:191–208
Stitt AW, McGoldrick C, Rice-McCaldin A, McCance DR, Glenn JV, Hsu DK, Liu FT, Thorpe SR, Gardiner TA (2005) Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes 54(3):785–794
Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A, Aiello LP, King GL (2011) Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care 34(4):968–974. https://doi.org/10.2337/dc10-1675
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7(2):168–181. https://doi.org/10.1038/nrd2467
Supuran CT, Scozzafava A (2007) Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 15(13):4336–4350. https://doi.org/10.1016/j.bmc.2007.04.020
Su L, Xiao H (2015) Inflammation in diabetes and cardiovascular disease: a new perspective on vitamin D. Cardiovasc Endocrinol 4(4):127–131. https://doi.org/10.1097/xce.0000000000000062
Su X, Sorenson CM, Sheibani N (2003) Isolation and characterization of murine retinal endothelial cells. Mol vis 9:171–178
Tang J, Du Y, Petrash JM, Sheibani N, Kern TS (2013) Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS ONE 8(4):e62081. https://doi.org/10.1371/journal.pone.0062081
The Diabetes Control and Complications Trial Research G (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329(14):977–986. https://doi.org/10.1056/nejm199309303291401
Tureci O, Sahin U, Vollmar E, Siemer S, Gottert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS (1998) Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci U S A 95(13):7608–7613
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(Pt 2):335–344. https://doi.org/10.1113/jphysiol.2003.049478
van Reyk DM, Gillies MC, Davies MJ (2003) The retina: oxidative stress and diabetes. Redox Rep 8(4):187–192
Vitale RM, Pedone C, Amodeo P, Antel J, Wurl M, Scozzafava A, Supuran CT, De Simone G (2007) Molecular modeling study for the binding of zonisamide and topiramate to the human mitochondrial carbonic anhydrase isoform VA. Bioorg Med Chem 15(12):4152–4158. https://doi.org/10.1016/j.bmc.2007.03.070
Vullo D, Franchi M, Gallori E, Antel J, Scozzafava A, Supuran CT (2004) Carbonic anhydrase inhibitors. Inhibition of mitochondrial isozyme V with aromatic and heterocyclic sulfonamides. J Med Chem 47(5):1272–1279. https://doi.org/10.1021/jm031057
Wallace DC (1992) Diseases of the mitochondrial DNA. Annu Rev Biochem 61:1175–1212. https://doi.org/10.1146/annurev.bi.61.070192.005523
Wang S, Gottlieb JL, Sorenson CM, Sheibani N (2009) Modulation of thrombospondin 1 and pigment epithelium-derived factor levels in vitreous fluid of patients with diabetes. Arch Ophthalmol 127(4):507–513
Weiwei Z, Hu R (2009) Targeting carbonic anhydrase to treat diabetic retinopathy: emerging evidences and encouraging results. Biochem Biophys Res Commun 390(3):368–371. https://doi.org/10.1016/j.bbrc.2009.10.031
Williams GR (1965) Dynamic aspects of the tricarboxylic acid cycle in isolated mitochondria. Can J Biochem 43:603–615
Winter K, Foster JG, Edwards GE, Holtum JA (1982) Intracellular localization of enzymes of carbon metabolism in mesembryanthemum crystallinum exhibiting C(3) photosynthetic characteristics or performing crassulacean acid metabolism. Plant Physiol 69(2):300–307
Winum JY, Thiry A, Cheikh KE, Dogne JM, Montero JL, Vullo D, Scozzafava A, Masereel B, Supuran CT (2007) Carbonic anhydrase inhibitors. Inhibition of isoforms I, II, IV, VA, VII, IX, and XIV with sulfonamides incorporating fructopyranose-thioureido tails. Bioorg Med Chem Lett 17(10):2685–2691. https://doi.org/10.1016/j.bmcl.2007.03.008
Wu MY, Yiang GT, Lai TT, Li CJ (2018) The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid Med Cell Longev 2018:3420187. https://doi.org/10.1155/2018/3420187
Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL (1994) Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes 43(9):1122–1129
Yamagishi S, Hsu CC, Taniguchi M, Harada S, Yamamoto Y, Ohsawa K, Kobayashi K, Yamamoto H (1995) Receptor-mediated toxicity to pericytes of advanced glycosylation end products: a possible mechanism of pericyte loss in diabetic microangiopathy. Biochem Biophys Res Commun 213(2):681–687
Yamagishi S, Inagaki Y, Amano S, Okamoto T, Takeuchi M, Makita Z (2002) Pigment epithelium-derived factor protects cultured retinal pericytes from advanced glycation end product-induced injury through its antioxidative properties. Biochem Biophys Res Commun 296(4):877–882
Zhang SX, Wang JJ, Dashti A, Wilson K, Zou MH, Szweda L, Ma JX, Lyons TJ (2008) Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J Mol Endocrinol 41(3):135–143
Acknowledgements
The work in NS lab is supported by an award from RPB to the Department of Ophthalmology and Visual Sciences, Retina Research Foundation, P30 EY016665, P30 CA014520, EPA 83573701, EY022883, and EY026078. NS is a recipient of RPB Stein Innovation Award. The authors wish to thank Dr. Gul Shah, Dr. Mahsa Ranji, and Dr. Christine Sorenson for their collaborations in a number of studies cited here and their long interest in carbonic anhydrases and oxidative stress in neuroinflammatory dysfunctions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gurel, Z., Sheibani, N. (2021). Potential of Carbonic Anhydrase Inhibitors in the Treatment of Oxidative Stress and Diabetes. In: Chegwidden, W.R., Carter, N.D. (eds) The Carbonic Anhydrases: Current and Emerging Therapeutic Targets. Progress in Drug Research, vol 75. Springer, Cham. https://doi.org/10.1007/978-3-030-79511-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-79511-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79510-8
Online ISBN: 978-3-030-79511-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)