Abstract
Thermophilic Campylobacter, in particular Campylobacter jejuni, C. coli and C. lari are the main relevant Campylobacter species for human infections. Due to their high capacity of genetic exchange by horizontal gene transfer (HGT), rapid adaptation to changing environmental and host conditions contribute to successful spreading and persistence of these foodborne pathogens. However, extensive HGT can exert dangerous side effects for the bacterium, such as the incorporation of gene fragments leading to disturbed gene functions. Here we discuss mechanisms of HGT, notably natural transformation, conjugation and bacteriophage transduction and limiting regulatory strategies of gene transfer. In particular, we summarize the current knowledge on how the DNA macromolecule is exchanged between single cells. Mechanisms to stimulate and to limit HGT obviously coevolved and maintained an optimal balance. Chromosomal rearrangements and incorporation of harmful mutations are risk factors for survival and can result in drastic loss of fitness. In Campylobacter, the restricted recognition and preferential uptake of free DNA from relatives are mediated by a short methylated DNA pattern and not by a classical DNA uptake sequence as found in other bacteria. A class two CRISPR-Cas system is present but also other DNases and restriction–modification systems appear to be important for Campylobacter genome integrity. Several lytic and integrated bacteriophages have been identified, which contribute to genome diversity. Furthermore, we focus on the impact of gene transfer on the spread of antibiotic resistance genes (resistome) and persistence factors. We discuss remaining open questions in the HGT field, supposed to be answered in the future by current technologies like whole-genome sequencing and single-cell approaches.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Horizontal gene transfer (HGT) is the exchange of genetic material and plays a major role in genetic diversity of pathogens (Lawrence 2005; Daubin and Szollosi 2016). Therefore, HGT in Campylobacter jejuni is thought to lead to host adaptation and fitness enhancement. There are three types of HGT, natural transformation, conjugation and phage transduction (Fig. 1). During natural transformation, free environmental DNA is taken up and incorporated into the genome upon homologous recombination or in case of plasmids by plasmid reconstitution and replication. Free DNA might occur in the environment by active secretion from bacterial cells or by cell lysis. Conjugation, however, is limited to DNA exchange between donor and recipient cells being in physical contact with each other. Transduction describes the genetic exchange mediated by bacteriophages. HGT in Campylobacter is the main driving force for the outstanding genetic diversity of this pathogen (Wilson et al. 2009; Sheppard et al. 2008). In Sect. 2, we discuss various HGT mechanisms in thermophilic Campylobacter, including C. jejuni, C. coli and C. lari, which are the Campylobacter species most frequently implicated in human gut disease.
Overview of horizontal gene transfer (HGT) mechanisms, genetic barriers and impact on pathogen adaptation. The three mechanisms of HGT are depicted for thermophilic Campylobacter spp. Natural transformation of free external DNA (in green) occurs via a type II secretion/T4 pili system, which displays homology to the competence machinery of Neisseria (detailed in Fig. 2). Transfer of plasmids via conjugation (in blue) is mediated by type IV secretion systems (T4SS) in direct cell–cell contact. Two main classes of bacteriophages of the family Myoviridae mediate genetic diversity by transduction (in red). The Fletcherviruses, group III, CP8-like phages depend on capsular polysaccharide (CPS) modifications as receptor for host entry. The Firehammer, group II, CP220-like phages need motile bacteria for infection. Campylobacter limits natural transformation by selection of DNA from relatives harboring a methylated RAm6ATTY profile, provided by activity of the CtsM methylase. Periplasmic nucleases and cytoplasmic restriction–modification systems as well as the CRISPR-Cas type II-C system provide additional barriers for incoming DNA. HGT leads to frequent homologous and rare non-homologous recombination of genetic material, acquisition of plasmids and/or rearrangements of chromosomal loci, which in turn shapes genome evolution. Hence, Campylobacter populations genetically diversify providing preadaptation to changing environments, such as presence of antimicrobials, switch of hosts and environmental stress, thereby enhancing the bacterium’s overall fitness for survival and transmission
However, genetic changes harbor the risk of harmful mutations or unfavorable chromosomal rearrangements for the bacteria. Therefore, mechanisms for the regulation of DNA entry and recombination into the bacterial chromosome co-evolved. CRISPR-Cas can be considered as the bacterial immune system protecting cells form invading bacteriophages or plasmids (Hille et al. 2018). However, other nucleases including restriction–modification systems play an important role for limiting harmful transfer of genetic material into the foodborne pathogen and are discussed in Sect. 3. Nevertheless, HGT bears the advantage of rapid host adaptation due to fitness enhancement and, e.g., spread of antibiotic resistances. Hence, in Sect. 4, we focus on the current knowledge of interspecies gene transfer and acquisition of novel beneficial genetic traits in thermophilic Campylobacter spp.
2 Mechanisms of Horizontal Gene Transfer
Most studies are based on classical approaches, in which HGT is followed using a selective marker and phenotypic characterization of resulting bacterial colonies after incorporation of the transferred marker gene (Table 1, first column). The enormous capacity of transfer of a chloramphenicol selection marker by natural transformation in C. jejuni was impressively shown by establishing a plate DNA transformation assays for screening mutants (Wiesner et al. 2003). The principle of the assay was the spreading of a countable number of C. jejuni cells on an agar plate, which had been overlaid by 2.5 µg of transforming DNA, harboring a given selection marker. After two days of growth, bacterial colonies were patched on agar plates with and without antibiotic. Intriguingly, the authors observed that nearly all colonies comprised transformed cells. Assuming growth from single cells to visible colonies of around 106-107 cells, natural transformation occurred within approximately 20–25 generations. Two days of incubation were sufficient to generate a bacterial population with adequate capacity of adaptive survival based on a former single cell. The final result of HGT is monitored in classical approaches but the readout cannot distinguish between different steps of gene transfer. Furthermore, in some settings in which the cells are exposed to stress conditions, the parameter colony-forming units (CFU) can be biased due to the fastidious nature of Campylobacter spp. and might not reflect full capacity of gene transfer.
Single-cell approaches have the advantage of dissecting different steps of HGT and to localize DNA uptake/transfer complexes (Table 1, second column). The detection and quantification of gene transfer are feasible at the level of single cells, displaying phenotypic heterogeneity. Parameters for induction of gene transfer can more directly be identified, since the assay does not depend on the complete process including the incorporation and expression of a marker gene. However, accessibility and, thus, visibility of DNA during the transfer event are limited. For example, covalently labeled DNA can only be followed into the periplasm and transfer of DNA into the cytoplasm is only indirectly monitored by disappearance of fluorescence of non-covalently labeled DNA (Stingl et al. 2010). For conjugation and transduction, DNA is steadily protected within biological compartments, and the detection by antibodies using transmission electron microscopy (TEM) is a stochastic event. Thus, differential approaches combined with the construction and characterization of mutants are necessary for complete monitoring and quantification of DNA transfer.
A recent approach focusses on whole-genome analysis in order to monitor the overall effects of HGT on population dynamics (Table 1, third column). Different platforms for whole-genome sequencing are used and quality as well as interpretation parameters are currently harmonized in order to optimally compare datasets of different laboratories. Ideally, all three approaches are combined to reveal the complete process and impact of HGT in the foodborne pathogen.
2.1 Natural Transformation and Uptake of Free DNA
Natural transformation was first discovered almost one century ago in Streptococcus pneumoniae, when phenotypic changes upon addition of heat-inactivated virulent bacteria to a recipient non-virulent culture were observed (Griffith 1928). Avery and colleagues (1944) pinpointed the transforming agent as DNA. The term “competence” depicts the state, in which cells are able to take up free DNA and naturally transform, i.e., integrate genetic material into their genome or replicate epichromosomal elements autonomously. Potential benefits of natural transformation include the repair of mutations by incoming homologous DNA and the acquisition of new genes and, therefore, new functions, e.g., antibiotic resistance genes or virulence factors. In addition, DNA might serve as nutrient supply by offering a reservoir for recycling of nucleotides. Besides extracellular DNA might serve as a matrix for the formation of biofilms and can enhance persistence of the pathogen outside the host (Feng et al. 2018; Svensson et al. 2014). For comprehensive reviews on natural transformation in other bacteria refer e. g. to Dubnau and Blokesch (2019) and Bakkali and colleagues (2013). Since uptake of foreign DNA might represent a danger of acquiring harmful mutations, competence development is usually a highly regulated process (Johnston et al. 2014). Only few information is available on parameters controlling competence development in Campylobacter spp. C. jejuni seems to show the highest transformation levels under optimal growth conditions, but transformation also occurred, when growth was restricted at higher pH (Vegge et al. 2012). However, it is unclear, if already expressed DNA uptake complexes still functioned under growth limiting conditions or if competence development still occurred. Prolonged incubation times in the presence of DNA were performed in this study, which do not allow distinguishing activity of DNA uptake complexes from transcriptional regulation of competence genes. Wilson and colleagues (2003) suggested that lower CO2 levels led to decreased competence in C. jejuni strains, although also here pH effects cannot be ruled out.
Since Campylobacter are Gram-negative bacteria, free DNA for natural transformation has to be transported i) over the outer membrane into the periplasm and ii) across the inner membrane into the cytoplasm. Campylobacter harbors gene homologues of a type II secretion/type IV pilus system that were shown to be essential for DNA uptake in other organisms (Table 2, Fig. 2) (Parkhill et al. 2000; Gundogdu et al. 2007). An at least 1000-fold reduction in transformation frequency was observed by Wiesner and colleagues (2003) using a transposon-based mutagenesis approach in eleven genes, nine of them were named Campylobacter transformation system (cts) genes (Table 2). Six of the genes are located in an operon, ctsF-ctsE-ctsX-ctsP-ctsD-ctsR. The remaining three cts genes, ctsG, ctsT and ctsW are separately located on the chromosome. CtsP and CtsE harbor nucleotide-binding sites (Walker A and B boxes) and are proper candidates for empowering uptake of the DNA macromolecule and/or assembly of a (pseudo-)pilus, like ComGA or PilF/T in B. subtilis or Neisseria, respectively (Beauchamp et al. 2015). CtsP physically interacts with the unique CtsX protein, both located in the membrane, while CtsE seems to be located in the soluble fraction (Beauchamp et al. 2015). Campylobacter recognizes DNA from relatives by using the methylated RAATTY site (see Sect. 3). In N. gonorrhoeae, PilQ constitutes the outer membrane pore (Drake and Koomey 1995), mediating entry of external DNA into the periplasm. C. jejuni harbors the pilQ homolog ctsD (Wiesner et al. 2003), which might have similar function as outer membrane DNA pore in C. jejuni. The genes ctsF, ctsG and ctsT have homology to comGB, comGC (pilE in Gram-negative bacteria) and comGD of B. subtilis, playing putative roles in function and assembly of the type IV (pseudo-)pilus system. In particular, it was suggested that ComGB displays an integral membrane protein forming the base for pilus assembly, with ComGC as major and ComGD as minor pilins (Chen et al. 2006). Retraction of DNA bound to type IV competence pili in Vibrio was recently demonstrated (Ellison et al. 2018). It remains to be shown, if a similar mechanism for “grabbing” external DNA is present in Campylobacter or if a pseudopilus is sufficient for DNA uptake as shown for Neisseria (Obergfell and Seifert 2016).
Working model of the DNA uptake complex for natural transformation in Campylobacter jejuni A compared to the system in Neisseria gonorrhoeae B Uptake of free external DNA probably occurs in two steps. Transport into the periplasm is mediated by a type II secretion/T4 pili system. Homology analysis suggests CtsD as the outer membrane porin. CtsG might be the major pilin but further pilin proteins CtsT and Cj1078 were identified in C. jejuni (see also Table 2). CtsF might form the basis for pilus/pseudopilus. CtsE and CtsP were proposed as ATPases, eventually empowering the DNA uptake process and/or pili assembly. The unique membrane protein CtsX was shown to interact with CtsP. The role of ComE as DNA-binding protein in the periplasm is enigmatic, since a homolog is lacking in C. coli and C. lari. ComEC appears to be the inner membrane channel as proposed for all competent bacteria, leading to import of single-stranded DNA into the cytoplasm, eventually empowered by PriA. In Neisseria, the minor pilin ComP recognizes a specific DNA uptake sequence (DUS) for selective uptake of DNA from relatives. In C. jejuni, the methylated RAm6ATTY motif is recognized by a yet unknown receptor. Methylation is mediated by the CtsM methylase. OM, outer membrane; IM, inner membrane; SAM, S-adenosylmethionine; ATP, adenosine triphosphate; dsDNA, double-stranded DNA; ssDNA, single-stranded DNA
In addition, transposon insertion in ceuB, which is located in an operon structure with ceuC, ceuD, ceuE, encoding the enterochelin uptake system important for iron acquisition, resulted in impaired natural transformation. Furthermore, also ctsW, proC and the downstream region of ansA led to reduced transformation rates (Wiesner et al. 2003). The role of these genes in natural transformation is still unknown. Fry and colleagues reported that a galE mutant, with defects in lipopolysaccharide (LPS) synthesis, showed a mild 20-fold reduction in DNA uptake and chromosomal integration, which might be indicative of LPS influencing the function of the DNA uptake machinery (Fry et al. 2000).
The periplasmic DNA-binding competence protein (Com) ComE was shown to facilitate DNA entry into the periplasm in Neisseria and Vibrio by generation of a pulling force on double-stranded (ds) DNA upon binding (Hepp and Maier 2016; Seitz et al. 2014). The role of the C. jejuni comE homolog (Cj0011c) has not been unraveled completely. Transformation rates in Cj0011c knockout mutants were only decreased by 10- to 50-fold (Jeon and Zhang 2007). Interestingly, C. coli, which displays similar gene homologs of a type II secretion/type IV pilus system as C. jejuni (Table 2), lacks a comE homolog (Meric et al. 2014). comE is also missing in C. lari (Table 2). Hence, in C. coli and C. lari a role of ComE in natural transformation can be ruled out.
Once the DNA reaches the periplasm, dsDNA has to be unzipped for import of single-stranded (ss) DNA into the cytoplasm mediated by the inner membrane channel ComEC in all so far known competent bacteria (Dubnau and Blokesch 2019). Absence of transformation activity in C. jejuni comEC (Cj1211) insertional mutants was demonstrated, whereas binding and uptake of radiolabeled DNA were not impaired (Jeon et al. 2008). Depending on homology, incoming DNA will be incorporated into the genome by homologous recombination. Site-directed homologous recombination of transformed plasmid DNA was observed with homologous regions of at least 286 bp, whereas 125-270 bp homology only led to a random and rare non-homologous insertion into the chromosome (Richardson and Park 1997). However, although non-homologous integration of DNA is infrequent, this mechanism guarantees the incorporation of completely novel genes.
C. jejuni strain 81–176 carries the plasmid pVir, encoding homologs to Helicobacter pylori cag pathogenicity island as well as homologs to type IV secretion systems (Bacon et al. 2002; Fischer et al. 2020). The role of pVir in natural transformation is not completely understood. Nevertheless, Bacon and colleagues (2000) showed an 80% reduction in transformation frequency in a comB3 mutant, whereas virB11 inactivation did not show a reduced transformation activity. Mutation of one of the glycosylation sites in the glycoprotein VirB10 or deletion of virB10 showed a mild ~ tenfold decrease in transformation efficiency (Larsen et al. 2004). Knockout of the N-linked protein glycosylation system (pgl), e.g., by deletion of pglB or pglE led to a drastic 10,000-fold decreased transformation rate (Larsen et al. 2004), suggesting that glycosylation of proteins is essential for natural transformation. Mutations in virD4 and comB1 led to wild-type transformation activity (Wiesner et al. 2003), thus, unlike the situation in the close relative H. pylori, the VirB/ComB system does not seem to play a major role for DNA uptake in C. jejuni.
2.2 Conjugative Gene Transfer
In conjugation processes, DNA is transferred from a donor to a recipient cell through cell-to-cell contact (Lederberg and Tatum 1946). To date 177 plasmid sequences from Campylobacter spp. are released at NCBI (https://www.ncbi.nlm.nih.gov/genome/browse#!/plasmids/campylobacter; accession on 22.09.2020). The size of currently identified C. jejuni and C. coli plasmids ranges from 1.3 to 190 kb. The presence of megaplasmids was shown in various strains from retail (Marasini and Fakhr 2016, 2014; Ghatak et al. 2017; Gunther et al. 2016). The transfer of plasmid-encoded antibiotic resistances in Campylobacter has frequently been reported (Taylor et al. 1981; Velazquez et al. 1995; Gibreel et al. 2004; Batchelor et al. 2004; Pratt and Korolik 2005; Zeng et al. 2015; Tang et al. 2017). Type-1 plasmids (pTet) harboring tetO are most prevalent in C. jejuni and C. coli (Schmidt-Ott et al. 2005; Marasini et al. 2018). Although only the tetO gene is representative for all pTet plasmids, most of them carry a VirB-type IV secretions system for conjugation. Type-2 plasmids were primarily found in C. coli strains, which are characterized to have a size between 24 and 32 kb and bear several trb genes for conjugative transfer as well as virD4, traI and traQ (Marasini et al. 2018).
The pVir plasmid of C. jejuni strain 81-176 mentioned above is categorized as the “prototype” of the type-3 plasmids. Small plasmids < 6 kb were categorized as type-4 plasmids despite absence of homologous genes shared between them. They contain genes with hypothetical function and replication initiator genes and await further investigations.
Pratt and Korolik (2005) showed that conjugation frequencies of a plasmids encoding tetO from donor strains to a recipient strain varied between ~10−8 and 10−6 within 6 h of mating. Interestingly, also co-transfer of a smaller plasmid was observed together with a larger plasmid conferring resistance to tetracycline (Pratt and Korolik 2005). Absence of conjugation in some strains was observed, indicative of barriers, e.g., restriction–modification systems and/or inability of plasmids to replicate in specific strains. Strain dependency of conjugation rates was identified in a different study, showing variations from 10−8 to 10−3 (Zeng et al. 2015). Hence, it might be concluded that natural transformation with transformation rates of ~ 10−3–10−2 is a more efficient way of HGT in Campylobacter spp. However, further studies are needed to collect more data on different field strains and to correlate in vitro with in vivo HGT frequencies.
Conjugation efficiency was induced 100–1000-fold in strains with low-frequency conjugation (LFC) upon 30 min heat shock at around 50 °C (Zeng et al. 2015). Recently, Zeng and colleagues (2018) identified the restriction–modification enzyme CjeI (Cj1051c) as crucial factor for reduced conjugation rate in the LFC strain NCTC11168. In high conjugation frequency (HCF) strains, 1000-fold reduced conjugation frequency was observed upon chromosomal complementation with cjeI. The cjeI mutants showed enhanced conjugation efficiency, which was nearly independent of heat shock, suggesting that CjeI was the heat-inactivated limiting factor of successful conjugational transfer of plasmids in LFC strains. It was previously observed that CjeI also restricted incoming DNA during natural transformation (Holt et al. 2012). Restriction barriers are discussed in more detail in Sect. 3.
Interestingly, unidirectional DNaseI-resistant conjugation-like transfer of a chromosomal resistance gene was observed from H. pylori to C. jejuni (Oyarzabal et al. 2007), demonstrating the potential of bacteria of the class Campylobacterales for genetic exchange (Fernandez-Gonzalez et al. 2014).
2.3 Phage Transduction and Genomic Rearrangements
Campylobacter bacteriophages have been isolated from diverse matrices, including food, animals and environments (for a recent review on isolation methods, see Jäckel et al. 2019), indicating that the pathogen is constantly exposed to phages in its natural habitat. Campylobacter bacteriophages were first reported in 1968 in C. fetus (formerly Vibrio fetus) upon induction of lytic phase by the bactericidal agent mitomycin C (Firehammer and Border 1968). For details on the application of bacteriophages for Campylobacter infection control, the reader should refer to Chap. 6 of this book.
Most sequenced Campylobacter phages (CP) belong to the family Myoviridae, displaying long contractile tails (Javed et al. 2014; NCBI Taxonomy Browser, accession 22.09.2020). They are categorized into two main groups, the Firehammervirus, group II, CP220-like and the Fletcherviruses, group III, CP8-like phages. Group I phages with large genomes of ~320 kb are, however, rare. DNA from Campylobacter phages was observed to be refractory to digestion by several restriction enzymes (Sails et al. 1998), which was recently attributed to complete exchange of deoxyguanosine (dG) by modified bases in phage DNA (Crippen et al. 2019).
In general, bacteriophage predation was shown to lead to chromosomal rearrangements in bacteria and, therefore, phages could also contribute to Campylobacter genomic evolution. For example, up to 590 kb in C. jejuni were inverted due to inversions caused by Mu-like phages (Scott et al. 2007). Interestingly, C. jejuni carrying the bacteriophage in its chromosome were resistant to infections by other bacteriophages but revealed an inefficient colonization of the chicken. Besides, the integration of phage-like elements into the genome can lead to genomic changes, visible by altered pulsed-field gel electrophoresis (PFGE) patterns of cleaved chromosomal DNA (Barton et al. 2007).
The process of phage transduction can be divided into several steps. Initially, the bacteriophage has to interact with a receptor on the bacterial cell. Principally, Campylobacter phage infection was shown to be either dependent on modifications of the capsular polysaccharides (Sorensen et al. 2011) or on motile flagella (Baldvinsson et al. 2014). Receptor-type dependency could be correlated with phage genus (Sorensen et al. 2015). While CP81-like Fletcherviruses were dependent on capsular polysaccharide (CPS), thereby unable to infect acapsular (ΔkpsM) mutants, the CP220-like Firehammerviruses were deficient of infecting non-motile (ΔmotA) C. jejuni strains. The receptor in C. jejuni NCTC 11168 for the Myoviridae phage F336 was shown to be an O-methyl phosphoramidate attached to 2-acetamido-2-deoxy-D-galactofuranose (GalfNAc) on the capsular polysaccharide (Sorensen et al. 2011). A frameshift in the phase variable homopolymeric G tract of gene Cj1421 resulting in a non-functional O-methyl phosphoramidate (MeOPN) transferase conferred resistance against phage F336. This is because the receptor is unavailable due to lack of receptor attachment to CPS. In addition, Cj1422, another phase variable gene, was shown to attach MeOPN to a heptose in CPS in C. jejuni, which confers resistance to F336 (Holst Sorensen et al. 2012; Aidley et al. 2017). The existence of further CPS receptors independent of MeOPN in CPS-dependent phages was suggested recently (Gencay et al. 2018).
In addition, it was shown that a conserved glycan-specific phage protein, Gp047 renamed FlaGrab, recognizes 7-acetamidino-modified pseudaminic acid residues on Campylobacter flagella, inhibiting bacterial growth (Javed et al. 2015). In particular, FlaGrab exposure led C. jejuni cells to downregulate expression of energy metabolism genes, which was dependent on a functional flagellar motor and was host strain-dependent, irrespective of the level of motility (Sacher et al. 2020). However, FlaGrab is also present in CPS-dependent phages, but is not part of the phage capsule. Thus, it was speculated that FlaGrab is not involved in phage entry, but presents an important protein in the phage lifecycle. It may either function as extracellular effector molecule upon phage-induced cell lysis, improving new infection by reduction of host motility or intracellularly during phage infection (Javed et al. 2015).
The transcriptional bacterial response upon infection of a CP8-like type-III phage NCTC 12673 revealed regulation of an unknown operon with some homology to T4 phage superinfection exclusion and antitoxin genes, as well as multidrug efflux pumps and oxidative stress defense genes (Sacher et al. 2018). Mutants of the cmeABC efflux pump were more susceptible for phage infections, while loss of catalase and superoxide dismutase genes led to enhanced phage resistance (Sacher et al. 2018). Thus, it seems that phage infection modulates the capacity of the host to resist antimicrobial treatment and oxidative stress, probably as part of phage–host dynamics. Interestingly, RidA, previously shown to play a role in flagella–flagella interactions due to regulation of flagellar glycan modification and motility (Reuter et al. 2015), was observed to also function in bacteriophage infectivity (Irons et al. 2019). However, the exact molecular mechanism is not yet clear. Taken together, more studies are clearly needed to fully understand Campylobacter phage lifecycle and the complex interaction with their host.
The ganglioside-like structures GM1 and GD1 generated by the cts-II-encoded sialyltransferase play a role in resistance against bacteriophages (Louwen et al. 2013). This was first suggested by the observation that isolates involved in Guillain-Barré syndrome induction showed lower susceptibility to a panel of 29 bacteriophages. Furthermore, a ΔctsII mutant showed increased susceptibility to bacteriophage than the wild-type bacteria. Bioinformatic screening revealed a correlation between the presence of ctsII and a degenerated CRISPR-Cas system (see also Sect. 3) in C. jejuni strains, indicating that virulence-associated ganglioside-like structures might serve as bacteriophage defense system.
While above we have discussed the current knowledge on lytic phages, also chromosomally integrated prophages have been described in various Campylobacter strains. For example, C. jejuni strain RM1221 carries four so-called Campylobacter jejuni-integrated elements (CJIEs), three of which (CJIE1, 2 and 4) seem to originate from phages (Barton et al. 2007) and the fourth (CJIE3) putatively from an integrated plasmid (Fouts et al. 2005; Parker et al. 2006). The Mu-like phage CJIE1 is integrated upstream of the argC gene, encoding an N-acetyl-γ-glutamyl-phosphate reductase. CJIE2 and CJIE4 are integrated at the 3’end of arginyl- and methionyl-tRNA genes. CJIE3 is integrated into the 3’ end of an arginyl-tRNA. CJIE1 encodes typical Mu and Mu-like phage proteins. CJIE2 and CJIE4 potentially encode methylases, endonucleases and repressors. CJIE1 was present among ~ 1/7 of Campylobacter isolates obtained from surveillance programs in Canada (Clark 2011). Most of these isolates were C. jejuni but CJIE1 was also present in C. coli and C. upsaliensis. The sequence and structure of the integrated CJIE1 varied, leading to protein alterations (Clark and Ng 2008). Furthermore, integration loci varied in different C. jejuni strains (Parker et al. 2006). Similarly to CJIE1, also CJIE2 and CJIE4 were inserted at different loci in the Campylobacter chromosome in different strains (Clark and Ng 2008). However, until now, induction of these CJIE prophages to lytic phase was unsuccessful (Clark and Ng 2008).
3 Barriers to Horizontal Gene Transfer
While HGT is crucial for the acquisition of novel genetic material and beneficial adaptation to changing environments, introgression of foreign DNA in bacterial genomes can also lead to tremendous fitness loss. The fact that Campylobacter is well protected by HGT barriers becomes obvious, since genetic manipulation of Campylobacter is hampered using constructs amplified in cloning strains of Escherichia coli (Gardner and Olson 2012). In the following, we address the aspect of barriers to HGT and focus on the CRISPR-Cas system and on other nucleases, including restriction–modification systems protecting Campylobacter against incoming foreign DNA. Furthermore, we address the question how C. jejuni can select for DNA of relatives, without using a classical DNA uptake sequence as demonstrated for other bacteria.
3.1 CRISPR-Cas and Nucleases
“Bacterial immunity” based on clustered regularly interspaces short palindromic repeats (CRISPR) and CRISPR-associated (Cas) proteins might be a powerful mechanism for restriction of horizontal gene transfer in Campylobacter. These systems are present in ~ 40% of complete bacterial and ~ 85% of archaeal genomes (Makarova et al. 2020). The principle is that incoming foreign DNA is memorized by incorporation of small fragments in CRISPR regions. Upon repeated entry, complementary CRISPR rRNAs (crRNA) in complex with Cas proteins target invading DNA for degradation. CRISPR-Cas systems are classified into two classes, six types and at least 33 subtypes (Makarova et al. 2020). While class 1 systems use multiple Cas proteins building up the effector complex, the class 2 system uses a single-protein effector, e.g., Cas9 in case of Campylobacter spp. Class 2 systems currently include three types and 17 subtypes. C. jejuni harbors a class 2, type II-C CRISPR-Cas system. It consists of the genes cas1, cas2 and cas9 as well as a trans-activating CRISPR RNA (TracrRNA). Cas1 and Cas2 are suggested to acquire and integrate new protospacers (Yosef et al. 2012). Cas9 participates in spacer acquisition (Heler et al. 2015; Wei et al. 2015). CRISPR loci are transcribed as a single pre-crRNA precursor, which is processed to crRNAs by the bacterial non-Cas RNase III in type II systems. In turn, crRNAs in complex with Cas9 silence invading plasmid or phage DNA, which bear sequence homology to the integrated spacer sequences. DNA strand breaks at stalled replication forks induce RecBCD-dependent spacer acquisition. In order to avoid autoimmunity, chromosomal loci were protected against spacer acquisition by relatively abundant Chi sites in E. coli, at which dsDNA break repair is stimulated in bacteria (Levy et al. 2015). However, self-DNA might be integrated into the CRISPR loci at very low frequency (Stern et al. 2010). Cas4-like proteins in Campylobacter bacteriophages were suggested to modify spacer element acquisition in favor of phage evasion due to preferential integration of host sequences in CRISPR loci (Hooton and Connerton 2014). Thus, coevolution of phages with the host leads to continuous modulation of genome dynamics.
The optimal size of the bacterial memory is dependent on the diversity of threats, i.e., phages. Since the effectiveness of response is dependent on the number/concentration of crRNA-Cas complexes with matching specificity, the depth of memory was proposed to be limited to 10–100 spacers in bacteria (Bradde et al. 2020). Based on the current database called CRISPRCasFinder, hosted at the University of Paris-Saclay, the numbers of predicted CRISPR loci in C. jejuni and C. coli range from 0 to 11 (median = 1; nCj = 207, nCc = 37, accession 22.09.2020 (at https://crisprcas.i2bc.paris-saclay.fr/MainDb/StrainList), harboring each one to multiple spacers (Grissa et al. 2007; Couvin et al. 2018). However, low transcription of crRNAs and TracrRNA was observed in C. jejuni RM1221 due to a stop-mutation in cas9 (Dugar et al. 2013). Thus, the authors suggested that absence of CRISPR loci or truncation of cas9 enabled acquisition of prophages or plasmids and that active CRISPR and mobile elements are mutually exclusive. Although Cas9 nucleases usually target dsDNA, a recent study demonstrated that in C. jejuni also endogenous ssRNA was targeted by CjCas9 (Dugar et al. 2018). Hence, it was proposed by the authors that CjCas9 may also serve to target RNA viruses or even regulate endogenous gene expression, which should be investigated in the future.
Apart from the CRISPR-Cas system, periplasmic nucleases were reported to degrade incoming genomic DNA, thereby inhibiting natural transformation. The periplasmic DNase, encoded by the dns gene (CJE0256) from Mu-like prophage CJIEI, inhibits natural transformation in RM1221 (Gaasbeek et al. 2009). Transformability of field strains correlated with presence or absence of dns. Homologs of DNA/RNA non-specific endonucleases were subsequently also detected on the prophages CJIE2 and CJIE4 and inhibition of natural transformation levels by around 30–40-fold were demonstrated (Gaasbeek et al. 2010).
3.2 Methylation-Dependent DNA Recognition
It has been reported long time ago that C. jejuni preferentially takes up DNA from siblings, although the mechanisms were completely unknown (Wang and Taylor 1990). However, C. jejuni does not have a typical DNA uptake sequence (DUS), like it was demonstrated for N. gonorrhoeae (Goodman and Scocca 1988), with the minor pilin protein ComP identified as specific receptor (Cehovin et al. 2013). Nevertheless, C. jejuni selects DNA of relatives and discriminates against foreign DNA. By single-molecule real-time sequencing (SMRT), a high degree of methylation of chromosomal DNA became apparent, with the RAATTY site being the only methylation site shared between C. coli BfR-CA-09557 and other C. jejuni strains (Zautner et al. 2015). By deletion of the respective methylase gene ctsM (named as Campylobacter transformation system methyltransferase), it was shown that C. jejuni recognizes the adenine N6 (exocyclic NH2-group at the sixth position of the purine ring) methylated RAATTY site of free external DNA as first step of natural transformation (Beauchamp et al. 2017). ctsM mutants were not impaired in DNA uptake, indicating that CtsM itself is not involved in recognition and/or transport of methylated DNA. The authors also demonstrated that E. coli plasmids could successfully be transformed into C. jejuni after methylation with the E. coli EcoRI methylase. In this case, one of the four RAATTY-sites, namely GAATTC is methylated, which was sufficient for DNA uptake in Campylobacter. This study presented a major advantage for future genetic manipulation of Campylobacter spp., since researchers can substantially improve genetic manipulation by methylation of plasmid constructs via commercially available EcoRI methylase prior to transformation in the respective Campylobacter host. The native EcoRI system is restricted to a special strain of E. coli and not ubiquitously found in this species, explaining that DNA from E. coli does not present a substrate for natural transformation of Campylobacter spp. It remains to be investigated, which components of the DNA uptake complex recognize the methylated RAATTY motif, in order to decipher the mechanism of selective DNA uptake in the foodborne pathogen.
Apart from CtsM as methylase, other restriction–modification systems are thought to constitute a genetic barrier for incoming DNA. The restriction–modification type IIG enzyme Cj1051c was shown to lower transformation efficiency using a C. jejuni derived plasmid by 1000-fold (Holt et al. 2012). Cj1051c was shown to also drastically reduce conjugation efficiency among C. jejuni strains (Zeng et al. 2018). Since Campylobacter genomes harbor diverse methylation profiles and various restriction-methylation genes, that are also strain-dependent (O’Loughlin et al. 2015; Zautner et al. 2015), it is expected that several other restriction–modification systems play crucial roles in establishing genetic barriers, even against relatives, favoring clonal spreading.
4 Impact of Gene Transfer on Campylobacter Fitness
As discussed above, during evolution Campylobacter spp. have developed powerful means for HGT and coevolved with incoming genetic material in order to balance the acquisition of novel material and putative detrimental effects. In the following, we address the beneficial impact of gene transfer and report on fitness advantages due to enormous genetic plasticity of the foodborne pathogen.
4.1 Spread of Resistomes and Persistence Factors
Human infections by Campylobacters are commonly caused by consumption and handling of raw poultry meat (for more details see Chap. 1 of this book). While most human campylobacteriosis cases are self-limiting, antibiotic treatment, in particular the use of macrolides or fluoroquinolones, was reported in around one-third of the patients (Rosner et al. 2017). The spread of antibiotic resistances by HGT enables preadaptation to changing environments and leads to diversification of the bacterial population. The observation of different resistances shared between C. jejuni and C. coli strains isolated from livestock, sewage and human disease indicated frequent spread of plasmids and multidrug-resistant genomic islands (MDRGIs) by HGT (Mourkas et al. 2019). Spread of antibiotic resistance between C. jejuni strains by natural transformation was reinforced in biofilms versus planktonic environments (Bae et al. 2014). For details on biofilm formation and quorum sensing, the reader should refer to Chap. 11 of this book. Biofilms contain extracellular DNA and are thought to convey enhanced persistence of host-associated pathogens in the environment. Their role in the dissemination of antibiotic resistances remains to be studied in more detail.
One of the well-known and the most prevailing resistance mechanisms of Campylobacter against macrolides in European strains is the point mutation A2075G in the 23S rRNA gene. This mutation is associated with a substantial decrease in bacterial fitness (Wang et al. 2014; Luangtongkum et al. 2012), probably leading to the currently observed low rates of macrolide resistance in Campylobacter spp. from livestock (EFSA 2020). Recently, C. jejuni and C. coli strains were isolated carrying the gene ermB, encoding an rRNA methylase, conferring resistance against macrolides in Asia (Qin et al. 2014; Du et al. 2018; Cheng et al. 2020; Liu et al. 2017), Europe (Florez-Cuadrado et al. 2016) and USA (Chen et al. 2018). Up to now, nine types of ermB-carrying MDRGI have been identified in Campylobacter spp. Besides ermB, these islands include resistances against aminoglycosides, such as gentamicin, kanamycin, streptomycin, spectinomycin or streptothricin, as well as ampicillins and tetracyclines (Wang et al. 2014; Florez-Cuadrado et al. 2016; Chen et al. 2018). C. coli strains were also identified, harboring ermB on different plasmids (Wang et al. 2014). The published NCBI sequences of Campylobacter ermB present four different allele variants. Comparative genome analysis revealed identical ermB sequences in Campylobacter, Streptococcus suis, Enterococcus faecium and Clostridium difficile isolates from different matrices (Florez-Cuadrado et al. 2017), suggesting multiple HGT events among different species. Especially the spread of macrolide resistance is of great danger, since macrolides are often drugs of choice to treat campylobacteriosis in humans (Rosner et al. 2017).
A variant of the multidrug efflux pump RE-CmeABC (for resistance-enhancing Campylobacter multidrug efflux system ABC), displaying sequence variation and enhanced expression due to a mutation in the promotor region, was shown to be spread via natural transformation and homologous recombination (Yao et al. 2016). This “super” pump conveys increased minimal inhibitory concentrations (MICs) against antimicrobials, such as ciprofloxacin, erythromycin, phenicols and tetracycline.
As long as the acquired antibiotic resistance determinant does not lead to fitness decrease, it can stably remain in a strain and is readily spread to other strains. For example, the transfer of tetO in C. jejuni was demonstrated to occur in vivo in chicken even without selection pressure (Avrain et al. 2004). This is especially important because it demonstrates that in case a long-term antimicrobial is discontinued, the resistance might persist and even spread to other strains. It is further stated that tetracyline resistance determinant, tetO, originated from Gram-positive cocci (Sougakoff et al. 1987) and kanamycin resistance seems to originate from Gram-positive cocci or from Enterobacteriaceae (Ouellette et al. 1987; Gibreel and Skold 1998). Apart from tetracycline resistance, resistance against (fluoro-)quinolones was observed to be another example of resistance determinant, not necessarily vanishing upon cease of antibiotic use. The resistance is conferred by the C257T point mutation in the gene encoding gyrase subunit A (gyrA). This point mutation was shown to even exert a fitness advantage on certain Campylobacter strains in the in vivo chicken gut environment (Luo et al. 2005). The spread of fluoroquinolone resistance in distinct clonal lineages might at least partially be explained by this fitness enhancement, although an additional selective pressure by antibiotic usage cannot be ruled out (Kovac et al. 2015; Leekitcharoenphon et al. 2018).
Not only the dissemination of antibiotic resistances bears risks for human health, but also the spread of bacterial persistence factors can increase the adaptive potential favoring the pathogens’ survival and transmission. However, since the function of gene variations is mostly unknown and it is expected that multiple gene exchanges synergistically lead to a beneficial adaptation, reports are scarce on the acquisition of novel traits other than antibiotic resistances. Mosaic sequence exchange in the highly similar flagellin genes flaA and flaB was observed on the intra- and intergenomic level (Wassenaar et al. 1995; Harrington et al. 1997). Campylobacter virulence is dependent on motility and, thus, a functional flagellar system. Hence, variations of the involved structural genes lead to variants, putatively evading host immune response.
Phongsisay and colleagues (2006) showed that human ganglioside-like structures, such as GM1, were readily transformable to strains not associated with Guillain-Barré syndrome induction in humans. The resulting transformants had acquired large DNA fragments and presented a high degree of genetic and phenotypic variation, corroborating the enormous potential of C. jejuni for genome plasticity upon natural transformation. Another interesting study highlighted successful HGT of genes with metabolic functions (Vorwerk et al. 2015). In particular, most Campylobacter strains are not capable of catabolizing glucose. Nevertheless, some C. coli strains harbor a genomic island, which allows using glucose as an energy source through the metabolic Entner–Doudoroff pathway. This locus was transferred between C. coli strains as well as between C. coli and C. jejuni, conferring glycolytic activity (Vorwerk et al. 2015), suggesting that this metabolic trait was acquired in order to optimize energy supply in distinct niches.
4.2 Interspecies Gene Transfer
As reported above, C. jejuni differentiates DNA of relatives by recognition of the methylated RAATTY profile, mediated by the N-adenine specific methylase CstM (Beauchamp et al. 2017). ctsM homologs are present in thermophilic Campylobacter spp., suggesting that gene transfer is enabled between different species. The manifestation of incoming DNA is further dependent on the degree of homology and of strain-specific restriction–modification systems as well as nucleases, which function as genetic barriers (see Sect. 3).
Genetic exchanges can also be analyzed using genome analysis of bacterial populations. The population structure of C. jejuni is different from C. coli even though their core genomes show a nucleotide sequence identity of ~ 85%, and they colonize similar habitats (Dingle et al. 2005). From nearly 3,000 MLST types, 11% of C. coli sequence types showed C. jejuni origin, vice versa this was only estimated for 0.6% of the C. jejuni types (Sheppard et al. 2008). This indicated a considerable but asymmetric gene flow between the two major thermophilic Campylobacter species. C. jejuni has a very diverse structure, with over 40 clonal complexes. In C. coli, only three different clades were identified (Sheppard et al. 2012). Clade 1 is predominantly found in clinical and animal farm samples and comprises the majority of all isolated and sequenced C. coli strains, whereas clade 2 and 3 were found in waterfowl and riparian environment. A genetic exchange between C. jejuni and C. coli of clade 1 was observed previously (Sheppard et al. 2011), while clade 2 and 3 were unaffected by C. jejuni introgression, probably due to separated niches and lack of contact with C. jejuni. A separation of individual clones with rare or no contact to others and a host tropism can explain why some strains isolated from the same host in different geographic location are more related than strains from different hosts (Sheppard and Maiden 2015). The study by Epping and colleagues (2020) analyzing whole-genome sequences of more than 490 C. jejuni strains obtained from Germany and Canada showed a strong host association and enables to further study host adaptation on the level of subsets of variant genes. For more details, the reader should refer to Chap. 3 of this book. Frequent HGT events might also give rise to a population of Campylobacter strains that are called “generalists,” able to colonize multiple hosts.
Introgression can occur as mosaic recombination of gene alleles. Consistent with asymmetric gene flow between the two species, the exchange from C. jejuni into C. coli was 17 times more frequently observed than from C. coli to C. jejuni (Sheppard et al. 2011). However, based on frequent genetic exchange, a convergence between the species C. coli and C. jejuni was postulated (Sheppard et al. 2008). For C. jejuni and C. coli, there are 44 clonal complexes and 11,111 sequence types defined (https://pubmlst.org, accession on 22.09.2020). Interestingly, nearly 40% of sequence types are not assigned to a clonal complex, demonstrating the diverse genome structure of these two major species. However, C. jejuni diversity is much greater than C. coli as defined by core genome phylogeny (Golz et al. 2020), with yet unknown reason.
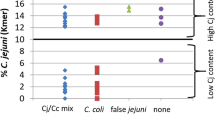
We have identified C. coli strains as a fraction of clade 1, which have undergone recent ongoing extended introgression by C. jejuni sequences (Golz et al. 2020). These strains were particularly isolated from chicken eggs, i.e., from fecal contamination on egg shells. K-mer analysis on whole-genome sequences revealed that these “hybrid” strains had incorporated up to 15% of genomic sequences from C. jejuni along the whole genome. However, a more in-depth analysis showed that recombination events were not random but followed a common pattern. In particular, C. jejuni introgression occurred in a common set of genes, implicated in stress defense. Hence, this genome alteration might represent a functional adaptation to survival in a harsh environment and confirms the enormous potential of natural transformation in shaping Campylobacter genomes.
5 Concluding Remarks
Due to high levels of genetic exchange by natural transformation, conjugation or transduction, Campylobacter shows an enormous genome diversity. This widens the pathogens adaptive potential and enables colonization of multiple hosts and successful survival in the environment, although the microaerobic bacterium is generally stress-sensitive and fastidious. Also, spread of antibiotic resistances endangers therapy options for treatment of campylobacteriosis (Oyarzabal and Backert 2012). The mechanisms of HGT in Campylobacter are yet poorly understood, and there is an urgent need to understand more in detail how the pathogen adapts by gene acquisition and/or gene variation. For example, open questions remain of how HGT is regulated in the pathogen, i.e., under which conditions gene transfer is most active and efficient. Once parameters are revealed that inhibit competence development and/or the function of HGT mechanisms, those critical elements could serve as target for the development of HGT inhibition. Especially in the context of control strategies such as chemical decontamination, bacteriophage treatment or vaccine development, it will be crucial to have a second-line strategy for prevention of pathogen adaptation. Therefore, the inhibition of HGT in Campylobacter is a promising approach in combating Campylobacter.
References
Aidley J, Sorensen MCH, Bayliss CD, Brondsted L (2017) Phage exposure causes dynamic shifts in the expression states of specific phase-variable genes of Campylobacter jejuni. Microbiology 163(6):911–919. https://doi.org/10.1099/mic.0.000470
Avery OT, Macleod CM, McCarty M (1944) Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med 79(2):137–158. https://doi.org/10.1084/jem.79.2.137
Avrain L, Vernozy-Rozand C, Kempf I (2004) Evidence for natural horizontal transfer of tetO gene between Campylobacter jejuni strains in chickens. J Appl Microbiol 97(1):134–140. https://doi.org/10.1111/j.1365-2672.2004.02306.x
Bacon DJ, Alm RA, Burr DH, Hu L, Kopecko DJ, Ewing CP, Trust TJ, Guerry P (2000) Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect Immun 68(8):4384–4390. https://doi.org/10.1128/iai.68.8.4384-4390.2000
Bacon DJ, Alm RA, Hu L, Hickey TE, Ewing CP, Batchelor RA, Trust TJ, Guerry P (2002) DNA sequence and mutational analyses of the pVir plasmid of Campylobacter jejuni 81-176. Infect Immun 70(11):6242–6250. https://doi.org/10.1128/iai.70.11.6242-6250.2002
Bae J, Oh E, Jeon B (2014) Enhanced transmission of antibiotic resistance in Campylobacter jejuni biofilms by natural transformation. Antimicrob Agents Chemother 58(12):7573–7575. https://doi.org/10.1128/AAC.04066-14
Bakkali M (2013) Could DNA uptake be a side effect of bacterial adhesion and twitching motility? Arch Microbiol 195(4):279–289. https://doi.org/10.1007/s00203-013-0870-1
Baldvinsson SB, Sorensen MC, Vegge CS, Clokie MR, Brondsted L (2014) Campylobacter jejuni motility is required for infection of the flagellotropic bacteriophage F341. Appl Environ Microbiol 80(22):7096–7106. https://doi.org/10.1128/AEM.02057-14
Barton C, Ng L-K, Tyler SD, Clark CG (2007) Temperate bacteriophages affect pulsed-field gel electrophoresis patterns of Campylobacter jejuni. J Clin Microbiol 45(2):386–391. https://doi.org/10.1128/JCM.01513-06
Batchelor RA, Pearson BM, Friis LM, Guerry P, Wells JM (2004) Nucleotide sequences and comparison of two large conjugative plasmids from different Campylobacter species. Microbiology 150(Pt 10):3507–3517. https://doi.org/10.1099/mic.0.27112-0
Beauchamp JM, Erfurt RS, DiRita VJ (2015) Characterization and localization of the Campylobacter jejuni transformation system proteins CtsE, CtsP, and CtsX. J Bacteriol 197(3):636–645. https://doi.org/10.1128/JB.02434-14
Beauchamp JM, Leveque RM, Dawid S, DiRita VJ (2017) Methylation-dependent DNA discrimination in natural transformation of Campylobacter jejuni. Proc Natl Acad Sci U S A 114(38):E8053–E8061. https://doi.org/10.1073/pnas.1703331114
Bradde S, Nourmohammad A, Goyal S, Balasubramanian V (2020) The size of the immune repertoire of bacteria. Proc Natl Acad Sci U S A 117(10):5144–5151. https://doi.org/10.1073/pnas.1903666117
Cehovin A, Simpson PJ, McDowell MA, Brown DR, Noschese R, Pallett M, Brady J, Baldwin GS, Lea SM, Matthews SJ, Pelicic V (2013) Specific DNA recognition mediated by a type IV pilin. Proc Natl Acad Sci U S A 110(8):3065–3070. https://doi.org/10.1073/pnas.1218832110
Chen I, Provvedi R, Dubnau D (2006) A macromolecular complex formed by a pilin-like protein in competent Bacillus subtilis. J Biol Chem 281(31):21720–21727. https://doi.org/10.1074/jbc.M604071200
Chen JC, Tagg KA, Joung YJ, Bennett C, Francois Watkins L, Eikmeier D, Folster JP (2018) Report of erm(B)(+) Campylobacter jejuni in the United States. Antimicrobial agents and chemotherapy 62(6). https://doi.org/10.1128/AAC.02615-17
Cheng Y, Zhang W, Lu Q, Wen G, Zhao Z, Luo Q, Shao H, Zhang T (2020) Point deletion or insertion in CmeR-Box, A2075G substitution in 23S rRNA, and presence of erm(B): are key factors of erythromycin resistance in Campylobacter jejuni and Campylobacter coli isolated from central China. Front Microbiol 11:203. https://doi.org/10.3389/fmicb.2020.00203
Clark CG (2011) Sequencing of CJIE1 prophages from Campylobacter jejuni isolates reveals the presence of inserted and (or) deleted genes. Can J Microbiol 57(10):795–808. https://doi.org/10.1139/w11-069
Clark CG, Ng L-K (2008) Sequence variability of Campylobacter temperate bacteriophages. BMC Microbiol 8(1):49. https://doi.org/10.1186/1471-2180-8-49
Couvin D, Bernheim A, Toffano-Nioche C, Touchon M, Michalik J, Neron B, Rocha EPC, Vergnaud G, Gautheret D, Pourcel C (2018) CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res 46(W1):W246–W251. https://doi.org/10.1093/nar/gky425
Crippen CS, Lee YJ, Hutinet G, Shajahan A, Sacher JC, Azadi P, de Crecy-Lagard V, Weigele PR, Szymanski CM (2019) Deoxyinosine and 7-Deaza-2-Deoxyguanosine as carriers of genetic information in the DNA of Campylobacter Viruses. J Virol 93(23). https://doi.org/10.1128/JVI.01111-19
Daubin V, Szollosi GJ (2016) Horizontal Gene transfer and the history of life. Cold Spring Harb Perspect Biol 8(4):a018036. https://doi.org/10.1101/cshperspect.a018036
Dingle KE, Colles FM, Falush D, Maiden MC (2005) Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J Clin Microbiol 43(1):340–347. https://doi.org/10.1128/JCM.43.1.340-347.2005
Drake SL, Koomey M (1995) The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol Microbiol 18(5):975–986. https://doi.org/10.1111/j.1365-2958.1995.18050975.x
Du Y, Wang C, Ye Y, Liu Y, Wang A, Li Y, Zhou X, Pan H, Zhang J, Xu X (2018) Molecular identification of multidrug-resistant Campylobacter species from diarrheal patients and poultry meat in Shanghai China. Front Microbiol 9:1642. https://doi.org/10.3389/fmicb.2018.01642
Dubnau D, Blokesch M (2019) Mechanisms of DNA uptake by naturally competent bacteria. Annu Rev Genet 53:217–237. https://doi.org/10.1146/annurev-genet-112618-043641
Dugar G, Herbig A, Forstner KU, Heidrich N, Reinhardt R, Nieselt K, Sharma CM (2013) High-resolution transcriptome maps reveal strain-specific regulatory features of multiple Campylobacter jejuni isolates. PLoS Genet 9(5):e1003495. https://doi.org/10.1371/journal.pgen.1003495
Dugar G, Leenay RT, Eisenbart SK, Bischler T, Aul BU, Beisel CL, Sharma CM (2018) CRISPR RNA-dependent binding and cleavage of endogenous RNAs by the Campylobacter jejuni Cas9. Mol Cell 69(5):893–905 e897. https://doi.org/10.1016/j.molcel.2018.01.032
EFSA (2020) The European union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA Journal 18(3):e06007. https://doi.org/10.2903/j.efsa.2020.6007
Ellison CK, Dalia TN, Vidal Ceballos A, Wang JC, Biais N, Brun YV, Dalia AB (2018) Retraction of DNA-bound type IV competence pili initiates DNA uptake during natural transformation in Vibrio cholerae. Nat Microbiol 3(7):773–780. https://doi.org/10.1038/s41564-018-0174-y
Epping L, Piro RM, Knüver MT, Borowiak M, Huber C, Thürmer A, Malorny B, Stingl K, Fruth A, Wieler LH, Semmler T (2020) Genome-wide identification of Host-Specificity of Campylobacter jejuni in Germany based on Whole Genome Data. In: 6th Joint conference of DGHM and VAAM, Leipzig, Germany
Feng J, Ma L, Nie J, Konkel ME, Lu X (2018) Environmental stress-induced bacterial lysis and extracellular DNA release contribute to Campylobacter jejuni biofilm formation. Appl Environ Microbiol 84(5). https://doi.org/10.1128/AEM.02068-17
Fernandez-Gonzalez E, Backert S (2014) DNA transfer in the gastric pathogen Helicobacter pylori. J Gastroenterol. 49(4):594–604. https://doi.org/10.1007/s00535-014-0938-y
Firehammer B, Border M (1968) Isolation of temperate bacteriophages from Vibrio fetus. Am J Vet Res 29(11):2229–2235
Fischer W, Tegtmeyer N, Stingl K, Backert S (2020) Four chromosomal type IV secretion systems in Helicobacter pylori: composition, structure and function. Front Microbiol 11:1592. https://doi.org/10.3389/fmicb.2020.01592
Florez-Cuadrado D, Ugarte-Ruiz M, Meric G, Quesada A, Porrero MC, Pascoe B, Saez-Llorente JL, Orozco GL, Dominguez L, Sheppard SK (2017) Genome comparison of erythromycin resistant Campylobacter from turkeys identifies hosts and pathways for horizontal spread of erm(B) Genes. Front Microbiol 8:2240. https://doi.org/10.3389/fmicb.2017.02240
Florez-Cuadrado D, Ugarte-Ruiz M, Quesada A, Palomo G, Dominguez L, Porrero MC (2016) Description of an erm(B)-carrying Campylobacter coli isolate in Europe. J Antimicrob Chemother 71(3):841–843. https://doi.org/10.1093/jac/dkv383
Fouts DE, Mongodin EF, Mandrell RE, Miller WG, Rasko DA, Ravel J, Brinkac LM, DeBoy RT, Parker CT, Daugherty SC, Dodson RJ, Durkin AS, Madupu R, Sullivan SA, Shetty JU, Ayodeji MA, Shvartsbeyn A, Schatz MC, Badger JH, Fraser CM, Nelson KE (2005) Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol 3(1):e15. https://doi.org/10.1371/journal.pbio.0030015
Fry BN, Feng S, Chen YY, Newell DG, Coloe PJ, Korolik V (2000) The galE gene of Campylobacter jejuni is involved in lipopolysaccharide synthesis and virulence. Infect Immun 68(5):2594–2601. https://doi.org/10.1128/iai.68.5.2594-2601.2000
Gaasbeek EJ, Wagenaar JA, Guilhabert MR, van Putten JP, Parker CT, van der Wal FJ (2010) Nucleases encoded by the integrated elements CJIE2 and CJIE4 inhibit natural transformation of Campylobacter jejuni. J Bacteriol 192(4):936–941. https://doi.org/10.1128/jb.00867-09
Gaasbeek EJ, Wagenaar JA, Guilhabert MR, Wosten MM, van Putten JP, van der Graaf-van Bloois L, Parker CT, van der Wal FJ (2009) A DNase encoded by integrated element CJIE1 inhibits natural transformation of Campylobacter jejuni. J Bacteriol 191(7):2296–2306. https://doi.org/10.1128/jb.01430-08
Gardner SP, Olson JW (2012) Barriers to horizontal gene transfer in Campylobacter jejuni. Adv Appl Microbiol 79:19–42. https://doi.org/10.1016/B978-0-12-394318-7.00002-4
Gencay YE, Sorensen MCH, Wenzel CQ, Szymanski CM, Brondsted L (2018) Phase variable expression of a single phage receptor in Campylobacter jejuni NCTC12662 influences sensitivity toward several diverse CPS-dependent phages. Front Microbiol 9:82. https://doi.org/10.3389/fmicb.2018.00082
Ghatak S, He Y, Reed S, Strobaugh T Jr, Irwin P (2017) Whole genome sequencing and analysis of Campylobacter coli YH502 from retail chicken reveals a plasmid-borne type VI secretion system. Genom Data 11:128–131. https://doi.org/10.1016/j.gdata.2017.02.005
Gibreel A, Skold O (1998) High-level resistance to trimethoprim in clinical isolates of Campylobacter jejuni by acquisition of foreign genes (dfr1 and dfr9) expressing drug-insensitive dihydrofolate reductases. Antimicrob Agents Chemother 42(12):3059–3064. https://doi.org/10.1128/AAC.42.12.3059
Gibreel A, Skold O, Taylor DE (2004) Characterization of plasmid-mediated aphA-3 kanamycin resistance in Campylobacter jejuni. Microb Drug Resist 10(2):98–105. https://doi.org/10.1089/1076629041310127
Golz JC, Epping L, Knuver MT, Borowiak M, Hartkopf F, Deneke C, Malorny B, Semmler T, Stingl K (2020) Whole genome sequencing reveals extended natural transformation in Campylobacter impacting diagnostics and the pathogens adaptive potential. Sci Rep 10(1):3686. https://doi.org/10.1038/s41598-020-60320-y
Goodman SD, Scocca JJ (1988) Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A 85(18):6982–6986. https://doi.org/10.1073/pnas.85.18.6982
Griffith F (1928) The significance of pneumococcal types. J Hyg (Lond) 27(2):113–159. https://doi.org/10.1017/s0022172400031879
Grissa I, Vergnaud G, Pourcel C (2007) The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinformatics 8:172. https://doi.org/10.1186/1471-2105-8-172
Gundogdu O, Bentley SD, Holden MT, Parkhill J, Dorrell N, Wren BW (2007) Re-annotation and re-analysis of the Campylobacter jejuni NCTC11168 genome sequence. BMC Genom 8:162. https://doi.org/10.1186/1471-2164-8-162
Gunther NWt, Reichenberger ER, Bono JL (2016) Complete genome sequence of UV-resistant Campylobacter jejuni RM3194, Including an 81.08-Kilobase Plasmid. Genome Announc 4(2). https://doi.org/10.1128/genomeA.00305-16
Harrington CS, Thomson-Carter FM, Carter PE (1997) Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J Clin Microbiol 35(9):2386–2392. https://doi.org/10.1128/JCM.35.9.2386-2392.1997
Heler R, Samai P, Modell JW, Weiner C, Goldberg GW, Bikard D, Marraffini LA (2015) Cas9 specifies functional viral targets during CRISPR-Cas adaptation. Nature 519(7542):199–202. https://doi.org/10.1038/nature14245
Hepp C, Maier B (2016) Kinetics of DNA uptake during transformation provide evidence for a translocation ratchet mechanism. Proc Natl Acad Sci U S A 113(44):12467–12472. https://doi.org/10.1073/pnas.1608110113
Hille F, Richter H, Wong SP, Bratovic M, Ressel S, Charpentier E (2018) The biology of CRISPR-Cas: backward and forward. Cell 172(6):1239–59. https://doi.org/10.1016/j.cell.2017.11.032
Holst Sorensen MC, van Alphen LB, Fodor C, Crowley SM, Christensen BB, Szymanski CM, Brondsted L (2012) Phase variable expression of capsular polysaccharide modifications allows Campylobacter jejuni to avoid bacteriophage infection in chickens. Front Cell Infect Microbiol 2:11. https://doi.org/10.3389/fcimb.2012.00011
Holt JP, Grant AJ, Coward C, Maskell DJ, Quinlan JJ (2012) Identification of Cj1051c as a major determinant for the restriction barrier of Campylobacter jejuni strain NCTC11168. Appl Environ Microbiol 78(22):7841–7848. https://doi.org/10.1128/AEM.01799-12
Hooton SP, Connerton IF (2014) Campylobacter jejuni acquire new host-derived CRISPR spacers when in association with bacteriophages harboring a CRISPR-like Cas4 protein. Front Microbiol 5:744. https://doi.org/10.3389/fmicb.2014.00744
Irons J, Sacher JC, Szymanski CM, Downs DM (2019) Cj1388 is a RidA homolog and is required for flagella biosynthesis and/or function in Campylobacter jejuni. Front Microbiol 10:2058. https://doi.org/10.3389/fmicb.2019.02058
Jäckel C, Hammerl JA, Hertwig S (2019) Campylobacter phage isolation and characterization: what we have learned so far. Methods Protoc 2(1). https://doi.org/10.3390/mps2010018
Javed MA, Ackermann HW, Azeredo J, Carvalho CM, Connerton I, Evoy S, Hammerl JA, Hertwig S, Lavigne R, Singh A, Szymanski CM, Timms A, Kropinski AM (2014) A suggested classification for two groups of Campylobacter myoviruses. Arch Virol 159(1):181–190. https://doi.org/10.1007/s00705-013-1788-2
Javed MA, Sacher JC, van Alphen LB, Patry RT, Szymanski CM (2015) A flagellar glycan-specific protein encoded by Campylobacter phages inhibits host cell growth. Viruses 7(12):6661–6674. https://doi.org/10.3390/v7122964
Jeon B, Muraoka W, Sahin O, Zhang Q (2008) Role of Cj1211 in natural transformation and transfer of antibiotic resistance determinants in Campylobacter jejuni. Antimicrob Agents Chemother 52(8):2699–2708. https://doi.org/10.1128/aac.01607-07
Jeon B, Zhang Q (2007) Cj0011c, a periplasmic single- and double-stranded DNA-binding protein, contributes to natural transformation in Campylobacter jejuni. J Bacteriol 189(20):7399–7407. https://doi.org/10.1128/jb.01012-07
Johnston C, Martin B, Fichant G, Polard P, Claverys JP (2014) Bacterial transformation: distribution, shared mechanisms and divergent control. Nat Rev Microbiol 12(3):181–196. https://doi.org/10.1038/nrmicro3199
Kovac J, Cadez N, Stessl B, Stingl K, Gruntar I, Ocepek M, Trkov M, Wagner M, Smole Mozina S (2015) High genetic similarity of ciprofloxacin-resistant Campylobacter jejuni in central Europe. Front Microbiol 6:1169. https://doi.org/10.3389/fmicb.2015.01169
Larsen JC, Szymanski C, Guerry P (2004) N-linked protein glycosylation is required for full competence in Campylobacter jejuni 81-176. J Bacteriol 186(19):6508–6514. https://doi.org/10.1128/jb.186.19.6508-6514.2004
Lawrence JG (2005) Horizontal and vertical gene transfer: the life history of pathogens. Contrib Microbiol 12:255–271. https://doi.org/10.1159/000081699
Lederberg J, Tatum EL (1946) Gene recombination in Escherichia coli. Nature 158(4016):558. https://doi.org/10.1038/158558a0
Leekitcharoenphon P, Garcia-Graells C, Botteldoorn N, Dierick K, Kempf I, Olkkola S, Rossi M, Nykäsenoja S, Malorny B, Stingl K (2018) Comparative genomics of quinolone-resistant and susceptible Campylobacter jejuni of poultry origin from major poultry producing European countries (GENCAMP). EFSA Support Publ 15(5):1398E. https://doi.org/10.2903/sp.efsa.2018.EN-1398
Levy A, Goren MG, Yosef I, Auster O, Manor M, Amitai G, Edgar R, Qimron U, Sorek R (2015) CRISPR adaptation biases explain preference for acquisition of foreign DNA. Nature 520(7548):505–510. https://doi.org/10.1038/nature14302
Liu D, Deng F, Gao Y, Yao H, Shen Z, Wu C, Wang Y, Shen J (2017) Dissemination of erm(B) and its associated multidrug-resistance genomic islands in Campylobacter from 2013 to 2015. Vet Microbiol 204:20–24. https://doi.org/10.1016/j.vetmic.2017.02.022
Louwen R, Horst-Kreft D, de Boer AG, van der Graaf L, de Knegt G, Hamersma M, Heikema AP, Timms AR, Jacobs BC, Wagenaar JA, Endtz HP, van der Oost J, Wells JM, Nieuwenhuis EE, van Vliet AH, Willemsen PT, van Baarlen P, van Belkum A (2013) A novel link between Campylobacter jejuni bacteriophage defence, virulence and Guillain-Barre syndrome. Eur J Clin Microbiol Infect Dis 32(2):207–226. https://doi.org/10.1007/s10096-012-1733-4
Luangtongkum T, Shen Z, Seng VW, Sahin O, Jeon B, Liu P, Zhang Q (2012) Impaired fitness and transmission of macrolide-resistant Campylobacter jejuni in its natural host. Antimicrob Agents Chemother 56(3):1300–1308. https://doi.org/10.1128/AAC.05516-11
Luo N, Pereira S, Sahin O, Lin J, Huang S, Michel L, Zhang Q (2005) Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc Natl Acad Sci U S A 102(3):541–546. https://doi.org/10.1073/pnas.0408966102
Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, Charpentier E, Cheng D, Haft DH, Horvath P, Moineau S, Mojica FJM, Scott D, Shah SA, Siksnys V, Terns MP, Venclovas C, White MF, Yakunin AF, Yan W, Zhang F, Garrett RA, Backofen R, van der Oost J, Barrangou R, Koonin EV (2020) Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol 18(2):67–83. https://doi.org/10.1038/s41579-019-0299-x
Marasini D, Fakhr MK (2014) Exploring PFGE for detecting large plasmids in Campylobacter jejuni and Campylobacter coli isolated from various retail meats. Pathogens 3(4):833–844. https://doi.org/10.3390/pathogens3040833
Marasini D, Fakhr MK (2016) Whole-genome sequencing of a Campylobacter jejuni strain isolated from retail chicken meat reveals the presence of a megaplasmid with Mu-like prophage and multidrug resistance genes. Genome Announc 4(3). https://doi.org/10.1128/genomeA.00460-16
Marasini D, Karki AB, Buchheim MA, Fakhr MK (2018) Phylogenetic relatedness among plasmids harbored by Campylobacter jejuni and Campylobacter coli isolated from retail meats. Front Microbiol 9:2167. https://doi.org/10.3389/fmicb.2018.02167
Meric G, Yahara K, Mageiros L, Pascoe B, Maiden MC, Jolley KA, Sheppard SK (2014) A reference pan-genome approach to comparative bacterial genomics: identification of novel epidemiological markers in pathogenic Campylobacter. PLoS ONE 9(3):e92798. https://doi.org/10.1371/journal.pone.0092798
Mourkas E, Florez-Cuadrado D, Pascoe B, Calland JK, Bayliss SC, Mageiros L, Meric G, Hitchings MD, Quesada A, Porrero C, Ugarte-Ruiz M, Gutierrez-Fernandez J, Dominguez L, Sheppard SK (2019) Gene pool transmission of multidrug resistance among Campylobacter from livestock, sewage and human disease. Environ Microbiol 21(12):4597–4613. https://doi.org/10.1111/1462-2920.14760
O’Loughlin JL, Eucker TP, Chavez JD, Samuelson DR, Neal-McKinney J, Gourley CR, Bruce JE, Konkel ME (2015) Analysis of the Campylobacter jejuni genome by SMRT DNA sequencing identifies restriction-modification motifs. PLoS ONE 10(2):e0118533. https://doi.org/10.1371/journal.pone.0118533
Obergfell KP, Seifert HS (2016) The pilin N-terminal domain maintains Neisseria gonorrhoeae transformation competence during pilus phase variation. PLoS Genet 12(5):e1006069. https://doi.org/10.1371/journal.pgen.1006069
Ouellette M, Gerbaud G, Lambert T, Courvalin P (1987) Acquisition by a Campylobacter-like strain of aphA-1, a kanamycin resistance determinant from members of the family Enterobacteriaceae. Antimicrob Agents Chemother 31(7):1021–1026. https://doi.org/10.1128/aac.31.7.1021
Oyarzabal OA, Backert S (2012) Microbial Food Safety: An Introduction (Food Science Text Series). Springer, Heidelberg
Oyarzabal OA, Rad R, Backert S (2007) Conjugative transfer of chromosomally encoded antibiotic resistance from Helicobacter pylori to Campylobacter jejuni. J Clin Microbiol 45(2):402–408. https://doi.org/10.1128/JCM.01456-06
Parker CT, Quiñones B, Miller WG, Horn ST, Mandrell RE (2006) Comparative genomic analysis of Campylobacter jejuni strains reveals diversity due to genomic elements similar to those present in C. jejuni strain RM1221. J Clinical Microbiol 44(11):4125–4135. https://doi.org/10.1128/JCM.01231-06
Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG (2000) The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403(6770):665–668. https://doi.org/10.1038/35001088
Phongsisay V, Perera VN, Fry BN (2006) Exchange of lipooligosaccharide synthesis genes creates potential Guillain-Barre syndrome-inducible strains of Campylobacter jejuni. Infect Immun 74(2):1368–1372. https://doi.org/10.1128/IAI.74.2.1368-1372.2006
Pratt A, Korolik V (2005) Tetracycline resistance of Australian Campylobacter jejuni and Campylobacter coli isolates. J Antimicrob Chemother 55(4):452–460. https://doi.org/10.1093/jac/dki040
Qin S, Wang Y, Zhang Q, Zhang M, Deng F, Shen Z, Wu C, Wang S, Zhang J, Shen J (2014) Report of ribosomal RNA methylase gene erm(B) in multidrug-resistant Campylobacter coli. J Antimicrob Chemother 69(4):964–968. https://doi.org/10.1093/jac/dkt492
Reuter M, Periago PM, Mulholland F, Brown HL, van Vliet AH (2015) A PAS domain-containing regulator controls flagella-flagella interactions in Campylobacter jejuni. Front Microbiol 6:770. https://doi.org/10.3389/fmicb.2015.00770
Richardson PT, Park SF (1997) Integration of heterologous plasmid DNA into multiple sites on the genome of Campylobacter coli following natural transformation. J Bacteriol 179(5):1809–1812. https://doi.org/10.1128/jb.179.5.1809-1812.1997
Rosner BM, Schielke A, Didelot X, Kops F, Breidenbach J, Willrich N, Golz G, Alter T, Stingl K, Josenhans C, Suerbaum S, Stark K (2017) A combined case-control and molecular source attribution study of human Campylobacter infections in Germany, 2011-2014. Sci Rep 7(1):5139. https://doi.org/10.1038/s41598-017-05227-x
Sacher JC, Flint A, Butcher J, Blasdel B, Reynolds HM, Lavigne R, Stintzi A, Szymanski CM (2018) Transcriptomic analysis of the Campylobacter jejuni response to T4-Like phage NCTC 12673 infection. Viruses 10(6). https://doi.org/10.3390/v10060332
Sacher JC, Shajahan A, Butcher J, Patry RT, Flint A, Hendrixson DR, Stintzi A, Azadi P, Szymanski CM (2020) Binding of phage-encoded FlaGrab to motile Campylobacter jejuni flagella inhibits growth, downregulates energy metabolism, and requires specific flagellar glycans. Front Microbiol 11:397. https://doi.org/10.3389/fmicb.2020.00397
Sails A, Wareing D, Bolton F, Fox A, Curry A (1998) Characterisation of 16 Campylobacter jejuni and C. coli typing bacteriophages. J Med Microbiol 47(2):123–128. https://doi.org/10.1099/00222615-47-2-123
Schmidt-Ott R, Pohl S, Burghard S, Weig M, Gross U (2005) Identification and characterization of a major subgroup of conjugative Campylobacter jejuni plasmids. J Infect 50(1):12–21. https://doi.org/10.1016/j.jinf.2004.02.013
Scott AE, Timms AR, Connerton PL, Carrillo CL, Radzum KA, Connerton IF (2007) Genome dynamics of Campylobacter jejuni in response to bacteriophage predation. PLoS Pathog 3(8):e119. https://doi.org/10.1371/journal.ppat.0030119
Seitz P, Pezeshgi Modarres H, Borgeaud S, Bulushev RD, Steinbock LJ, Radenovic A, Dal Peraro M, Blokesch M (2014) ComEA is essential for the transfer of external DNA into the periplasm in naturally transformable Vibrio cholerae cells. PLoS Genet 10(1):e1004066. https://doi.org/10.1371/journal.pgen.1004066
Sheppard SK, Jolley KA, Maiden MC (2012) A Gene-By-Gene Approach to Bacterial Population Genomics: Whole Genome MLST of Campylobacter. Genes (Basel) 3(2):261–277. https://doi.org/10.3390/genes3020261
Sheppard SK, Maiden MC (2015) The evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb Perspect Biol 7(8):a018119. https://doi.org/10.1101/cshperspect.a018119
Sheppard SK, McCarthy ND, Falush D, Maiden MC (2008) Convergence of Campylobacter species: implications for bacterial evolution. Science 320(5873):237–239. https://doi.org/10.1126/science.1155532
Sheppard SK, McCarthy ND, Jolley KA, Maiden MC (2011) Introgression in the genus Campylobacter: generation and spread of mosaic alleles. Microbiology 157(Pt 4):1066–1074. https://doi.org/10.1099/mic.0.045153-0
Sorensen MC, Gencay YE, Birk T, Baldvinsson SB, Jackel C, Hammerl JA, Vegge CS, Neve H, Brondsted L (2015) Primary isolation strain determines both phage type and receptors recognised by Campylobacter jejuni bacteriophages. PLoS ONE 10(1):e0116287. https://doi.org/10.1371/journal.pone.0116287
Sorensen MC, van Alphen LB, Harboe A, Li J, Christensen BB, Szymanski CM, Brondsted L (2011) Bacteriophage F336 recognizes the capsular phosphoramidate modification of Campylobacter jejuni NCTC11168. J Bacteriol 193(23):6742–6749. https://doi.org/10.1128/JB.05276-11
Sougakoff W, Papadopoulou B, Nordmann P, Courvalin P (1987) Nucleotide sequence and distribution of gene tetO encoding tetracycline resistance in Campylobacter coli. FEMS Microbiol Lett 44(1):153–159. https://doi.org/10.1111/j.1574-6968.1987.tb02260.x
Stern A, Keren L, Wurtzel O, Amitai G, Sorek R (2010) Self-targeting by CRISPR: gene regulation or autoimmunity? Trends Genet 26(8):335–340. https://doi.org/10.1016/j.tig.2010.05.008
Stingl K, Muller S, Scheidgen-Kleyboldt G, Clausen M, Maier B (2010) Composite system mediates two-step DNA uptake into Helicobacter pylori. Proc Natl Acad Sci U S A 107(3):1184–1189. https://doi.org/10.1073/pnas.0909955107
Svensson SL, Pryjma M, Gaynor EC (2014) Flagella-mediated adhesion and extracellular DNA release contribute to biofilm formation and stress tolerance of Campylobacter jejuni. PLoS ONE 9(8):e106063. https://doi.org/10.1371/journal.pone.0106063
Tang Y, Dai L, Sahin O, Wu Z, Liu M, Zhang Q (2017) Emergence of a plasmid-borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter. J Antimicrob Chemother 72(6):1581–1588. https://doi.org/10.1093/jac/dkx023
Taylor DE, De Grandis SA, Karmali MA, Fleming PC (1981) Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother 19(5):831–835. https://doi.org/10.1128/aac.19.5.831
Vegge CS, Brondsted L, Ligowska-Marzeta M, Ingmer H (2012) Natural transformation of Campylobacter jejuni occurs beyond limits of growth. PLoS ONE 7(9):e45467. https://doi.org/10.1371/journal.pone.0045467
Velazquez JB, Jimenez A, Chomon B, Villa TG (1995) Incidence and transmission of antibiotic resistance in Campylobacter jejuni and Campylobacter coli. J Antimicrob Chemother 35(1):173–178. https://doi.org/10.1093/jac/35.1.173
Vorwerk H, Huber C, Mohr J, Bunk B, Bhuju S, Wensel O, Sproer C, Fruth A, Flieger A, Schmidt-Hohagen K, Schomburg D, Eisenreich W, Hofreuter D (2015) A transferable plasticity region in Campylobacter coli allows isolates of an otherwise non-glycolytic food-borne pathogen to catabolize glucose. Mol Microbiol 98(5):809–830. https://doi.org/10.1111/mmi.13159
Wang Y, Taylor DE (1990) Natural transformation in Campylobacter species. J Bacteriol 172(2):949–955. https://doi.org/10.1128/jb.172.2.949-955.1990
Wang Y, Zhang M, Deng F, Shen Z, Wu C, Zhang J, Zhang Q, Shen J (2014) Emergence of multidrug-resistant Campylobacter species isolates with a horizontally acquired rRNA methylase. Antimicrob Agents Chemother 58(9):5405–5412. https://doi.org/10.1128/AAC.03039-14
Wassenaar TM, Fry BN, van der Zeijst BA (1995) Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer. Microbiology 141(Pt 1):95–101. https://doi.org/10.1099/00221287-141-1-95
Wei Y, Terns RM, Terns MP (2015) Cas9 function and host genome sampling in Type II-A CRISPR-Cas adaptation. Genes Dev 29(4):356–361. https://doi.org/10.1101/gad.257550.114
Wiesner RS, Hendrixson DR, DiRita VJ (2003) Natural Transformation of Campylobacter jejuni Requires Components of a Type II Secretion System. J Bacteriol 185(18):5408–5418. https://doi.org/10.1128/jb.185.18.5408-5418.2003
Wilson DJ, Gabriel E, Leatherbarrow AJ, Cheesbrough J, Gee S, Bolton E, Fox A, Hart CA, Diggle PJ, Fearnhead P (2009) Rapid evolution and the importance of recombination to the gastroenteric pathogen Campylobacter jejuni. Mol Biol Evol 26(2):385–397. https://doi.org/10.1093/molbev/msn264
Wilson DL, Bell JA, Young VB, Wilder SR, Mansfield LS, Linz JE (2003) Variation of the natural transformation frequency of Campylobacter jejuni in liquid shake culture. Microbiology 149(Pt 12):3603–3615. https://doi.org/10.1099/mic.0.26531-0
Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su CC, Wang B, Wang S, Wu C, Yu EW, Zhang Q, Shen J (2016) Emergence of a potent multidrug efflux pump variant that enhances Campylobacter resistance to multiple antibiotics. mBio 7(5). https://doi.org/10.1128/mBio.01543-16
Yosef I, Goren MG, Qimron U (2012) Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res 40(12):5569–5576. https://doi.org/10.1093/nar/gks216
Zautner AE, Goldschmidt AM, Thurmer A, Schuldes J, Bader O, Lugert R, Gross U, Stingl K, Salinas G, Lingner T (2015) SMRT sequencing of the Campylobacter coli BfR-CA-9557 genome sequence reveals unique methylation motifs. BMC Genom 16:1088. https://doi.org/10.1186/s12864-015-2317-3
Zeng X, Ardeshna D, Lin J (2015) Heat shock-enhanced conjugation efficiency in standard Campylobacter jejuni strains. Appl Environ Microbiol 81(13):4546–4552. https://doi.org/10.1128/aem.00346-15
Zeng X, Wu Z, Zhang Q, Lin J (2018) A cotransformation method to identify a restriction-modification enzyme that reduces conjugation efficiency in Campylobacter jejuni. Appl Environ Microbiol 84(23). https://doi.org/10.1128/AEM.02004-18
Acknowledgements
This work is supported by the German Federal Ministry of Education and Research (BMBF) in frame of the zoonoses research consortium PAC-CAMPY to K.S. (project IP3/01KI1725B).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Golz, J.C., Stingl, K. (2021). Natural Competence and Horizontal Gene Transfer in Campylobacter. In: Backert, S. (eds) Fighting Campylobacter Infections. Current Topics in Microbiology and Immunology, vol 431. Springer, Cham. https://doi.org/10.1007/978-3-030-65481-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-65481-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65480-1
Online ISBN: 978-3-030-65481-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)