Abstract
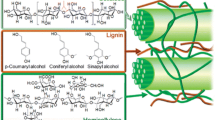
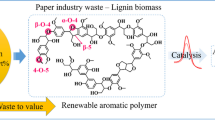
Lignin, a major component of lignocellulose, constitutes the largest source for the production of aromatic building blocks, and offers considerable potential to serve as an entry to biobased products. During the past decades, substantial research activity has been noted in the pursuit of sustainable catalytic methods for accessing lignin-derived platform chemicals. However, the highly cross-linked and irregular structure has hindered the development of efficient and predictable deconstruction strategies from this renewable source. A majority of the developed methods for lignin depolymerization require harsh conditions including high-pressure atmospheres of gases, high temperatures, and prolonged reaction times. Considering the recent renewed interest in photocatalysis as a means for the mild and operationally simple generation of open-shell intermediates, it is not remarkable that such reaction manifolds have also been implemented towards lignin depolymerization. The tremendous potential of visible light photocatalysis for harnessing sustainable energy sources and reducing waste streams will certainly have an enduring impact in future green chemistry innovation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abas, N., Kalair, A., Khan, N.: Review of fossil fuels and future energy technologies. Futures. 69, 31–49 (2015)
Alonso, D.M., Bond, J.Q., Dumesic, J.A.: Catalytic conversion of biomass to biofuels. Green Chem. 12, 1493–1513 (2010)
Mettler, M.S., Vlachos, D.G., Dauenhauer, P.J.: Top ten fundamental challenges of biomass pyrolysis for biofuels. Energy Environ. Sci. 5, 7797–7809 (2012)
Barta, K., Ford, P.C.: Catalytic conversion of nonfood Woody biomass solids to organic liquids. Acc. Chem. Res. 47, 1503–1512 (2014)
Besson, M., Gallezot, P., Pinel, C.: Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 114, 1827–1870 (2014)
Obama, B.: The irreversible momentum of clean energy. Science. 355, 126–129 (2017)
Monthly Energy Review – October 2018
Perlack, R.D., Wright, L.L., Turhollow, A.F., Graham, R.L., Stokes, B.J., Erbach, D.C.: Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply, p. 1216415 (2005)
McCarthy, J.L., Islam, A.: Lignin chemistry, technology, and utilization: a brief history. In: Lignin: Historical, Biological, and Materials Perspectives ACS Symposium Series, vol. 742, pp. 2–99. American Chemical Society (1999)
Rinaldi, R., Jastrzebski, R., Clough, M.T., Ralph, J., Kennema, M., Bruijnincx, P.C.A., Weckhuysen, B.M.: Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 55, 8164–8215 (2016)
Kärkäs, M.D., Matsuura, B.S., Monos, T.M., Magallanes, G., Stephenson, C.R.J.: Transition-metal catalyzed valorization of lignin: the key to a sustainable carbon-neutral future. Org. Biomol. Chem. 14, 1853–1914 (2016)
de Jong, E.; Jungmeier, G. Chapter 1 - Biorefinery concepts in comparison to petrochemical refineries. In Industrial Biorefineries & White Biotechnology; Pandey, A., Höfer, R., Taherzadeh, M., Nampoothiri, K. M., Larroche, C., Eds.; Elsevier: Amsterdam, 2015; pp. 3–33
Ragauskas, A.J., Beckham, G.T., Biddy, M.J., Chandra, R., Chen, F., Davis, M.F., Davison, B.H., Dixon, R.A., Gilna, P., Keller, M., et al.: Lignin valorization: improving lignin processing in the biorefinery. Science. 344, 1246843 (2014)
Ralph, J., Peng, J., Lu, F., Hatfield, R.D., Helm, R.F.: Are lignins optically active? J. Agric. Food Chem. 47, 2991–2996 (1999)
Crestini, C., Melone, F., Sette, M., Saladino, R.: Milled wood lignin: a linear oligomer. Biomacromolecules. 12, 3928–3935 (2011)
Zakzeski, J., Bruijnincx, P.C.A., Jongerius, A.L., Weckhuysen, B.M.: The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010)
Galkin, M.V., Samec, J.S.M.: Lignin valorization through catalytic lignocellulose fractionation: a fundamental platform for the future biorefinery. ChemSusChem. 9, 1544–1558 (2016)
Shuai, L., Talebi Amiri, M., Luterbacher, J.S.: The influence of interunit carbon–carbon linkages during lignin upgrading. Curr. Opin. Green Sustain. Chem. 2, 59–63 (2016)
Schutyser, W., Renders, T., Van den Bosch, S., Koelewijn, S.-F., Beckham, G.T., Sels, B.F.: Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 47, 852–908 (2018)
Shuai, L., Amiri, M.A., Questell-Santiago, Y.M., Héroguel, F., Li, Y., Kim, H., Meilan, R., Chapple, C., Ralph, J., Luterbacher, J.S.: Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science. 354, 329–333 (2016)
Lan, W., Amiri, M.T., Hunston, C.M., Luterbacher, J.S.: Protection group effects during α,γ-diol lignin stabilization promote high-selectivity monomer production. Angew. Chem. Int. Ed. 57, 1356–1360 (2018)
Kärkäs, M.D.: Lignin hydrogenolysis: improving lignin disassembly through formaldehyde stabilization. ChemSusChem. 10, 2111–2115 (2017)
For an overview on stabilization strategies in biomass depolymerization, see: Questell-Santiago, Y.M., Galkin, M.V., Barta, K., Luterbacher, J.S.: Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 4, 311–330 (2020)
Fargues, C., Mathias, Á., Rodrigues, A.: Kinetics of vanillin production from kraft lignin oxidation. Ind. Eng. Chem. Res. 35, 28–36 (1996)
Bauer, S., Sorek, H., Mitchell, V.D., Ibáñez, A.B.., Wemmer, D.E.: Characterization of Miscanthus giganteus lignin isolated by ethanol organosolv process under reflux condition. J. Agric. Food Chem. 60, 8203–8212 (2012)
Das, A., Rahimi, A., Ulbrich, A., Alherech, M., Motagamwala, A.H., Bhalla, A., da Costa Sousa, L., Balan, V., Dumesic, J.A., Hegg, E.L., et al.: Lignin conversion to low-molecular-weight aromatics via an aerobic oxidation-hydrolysis sequence: comparison of different lignin sources. ACS Sustain. Chem. Eng. 6, 3367–3374 (2018)
Holladay, J.E., White, J.F., Bozell, J.J., Johnson, D.: Top value-added chemicals from biomass - volume II–results of screening for potential candidates from biorefinery lignin. PNNL-16983, Oak Ridge, TN, October 2007
Safari, F., Tavasoli, A., Ataei, A., Choi, J.-K.: Hydrogen and syngas production from gasification of lignocellulosic biomass in supercritical water media. Int. J. Recycl. Org. Waste Agricult. 4, 121–125 (2015)
Son, S., Toste, F.D.: Non-oxidative vanadium-catalyzed C–O bond cleavage: application to degradation of lignin model compounds. Angew. Chem. Int. Ed. 49, 3791–3794 (2010)
Chan, J.M.W., Bauer, S., Sorek, H., Sreekumar, S., Wang, K., Toste, F.D.: Studies on the vanadium-catalyzed nonoxidative depolymerization of Miscanthus giganteus-derived lignin. ACS Catal. 3, 1369–1377 (2013)
Anderson, E., Arundale, R., Maughan, M., Oladeinde, A., Wycislo, A., Voigt, T.: Growth and agronomy of Miscanthus x giganteus for biomass production. Biofuels. 2, 71–87 (2011)
Dunleavy, J.: Final analysis: sulfur as a catalyst poison. Platin. Met. Rev. 50, 110 (2006)
Kavarnos, G.J., Turro, N.J.: Photosensitization by reversible electron transfer: theories, experimental evidence, and examples. Chem. Rev. 86, 401–449 (1986)
Turro, N.J., Ramamurthy, V., Scaiano, J.C.: Modern Molecular Photochemistry of Organic Molecules. University Science Books, Sausalito, California (2010)
For recent reviews on visible light photoredox catalysis, see: (a) Shaw, M.H., Twilton, J., MacMillan, D.W.C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016). (b) Kärkäs, M.D., Porco, J.A. Jr., Stephenson, C.R.J.: Photochemical approaches to complex Chemotypes: applications in natural product synthesis. Chem. Rev. 116, 9683–9747 (2016). (c) Romero, N.A., Nicewicz, D.A.: Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016). (d) Kärkäs, M.D.: Photochemical generation of nitrogen-centered amidyl, hydrazonyl, and imidyl radicals: methodology developments and catalytic applications. ACS Catal. 7, 4999–5022 (2017). (e) Larsen, C.B., Wenger, O.S.: Photoredox catalysis with metal complexes made from earth-abundant elements. Chem. Eur. J. 24, 2039–2058 (2018). (f) Marzo, L., Pagire, S.K., Reiser, O., König, B.: Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 57, 10034–10072 (2018). (g) Silvi, M., Melchiorre, P.: Enhancing the potential of enantioselective organocatalysis with light. Nature. 554, 41–49 (2018). (h) Wang, C.-S., Dixneuf, P. H., Soulé, J.-F.: Photoredox catalysis for building C–C bonds from C(sp2)–H bonds. Chem. Rev. 118, 7532–7585 (2018). (i) Sun, A.C., McAtee, R.C., McClain, E.J., Stephenson, C.R.J.: Advancements in visible-light-enabled radical C(sp)2–H alkylation of (hetero)arenes. Synthesis. https://doi.org/10.1055/s-0037-1611658
For a review on the use of radicals in natural product synthesis, see: Romero, K.J., Galliher, M.S., Pratt, D.A., Stephenson, C.R.J.: Radicals in natural product synthesis. Chem. Soc. Rev. 47, 7851–7866 (2018)
For a review on palladium-catalyzed direct dioxygen-coupled catalytic turnover, see: Stahl, S.S.: Palladium oxidase catalysis: selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem. Int. Ed. 43, 3400–3420 (2004)
For leading reviews on aerobic oxidation, see: (a) Piera, J., Bäckvall, J.-E.: Catalytic oxidation of organic substrates by molecular oxygen and hydrogen peroxide by multistep electron transfer—a biomimetic approach. Angew. Chem. Int. Ed. 47, 3506–3523 (2008). (b) Campbell, A.N., Stahl, S.S.: Overcoming the “oxidant problem”: strategies to use O2 as the oxidant in organometallic C–H oxidation reactions catalyzed by Pd (and Cu). Acc. Chem. Res. 45, 851–863 (2012). (c) Shi, Z., Zhang, C., Tang, C., Jiao, N.: Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev. 41, 3381–3430 (2012). (d) Parmeggiani, C., Cardona, F.: Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 14, 547–564 (2012). (e) Allen, S.E., Walvoord, R.R., Padilla-Salinas, R., Kozlowski, M.C.: Aerobic copper-catalyzed organic reactions. Chem. Rev. 113, 6234–6458 (2013). (f) Cao, Q., Dornan, L.M., Rogan, L., Hughes, N.L., Muldoon, M.J.: Aerobic oxidation catalysis with stable radicals. Chem. Commun. 50, 4524–4543 (2014). (g) Ryland, B.L., Stahl, S.S.: Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems. Angew. Chem. Int. Ed. 53, 8824–8838 (2014). (h) Wang, D., Weinstein, A.B.., White, P.B., Stahl, S.S.: Ligand-promoted palladium-catalyzed aerobic oxidation reactions. Chem. Rev. 118, 2636–2679 (2018)
Patai, S., Rappoport, Z. (eds.): The Chemistry of Quinonoid Compounds, vol. 2. Wiley-Interscience, Bath (1988)
For reviews on quinone-mediated reactions, see: (a) Walker, D., Hiebert, J.D.: 2,3-dichloro-5,6-dicyanobenzoquinone and its reactions. Chem. Rev. 67, 153–195 (1967). (b) Wendlandt, A.E., Stahl, S.S.: Quinone-catalyzed selective oxidation of organic molecules. Angew. Chem. Int. Ed. 54, 14638–14658 (2015)
Wendlandt, A.E., Stahl, S.S.: Quinones in hydrogen peroxide synthesis and catalytic aerobic oxidation reactions. In: Stahl, S.S., Alsters, P.L. (eds.) Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim (2016)
For examples on the use of quinones in natural product and pharmaceutical syntheses, see: (a) Yu, J., Wearing, X.Z., Cook, J.M.: The first enantiospecific total synthesis of a C-quaternary voachalotine alkaloid, (+)-dehydrovoachalotine. Tetrahedron Lett. 45, 3937–3940 (2004). (b) Mickel, S.J., Sedelmeier, G.H., Niederer, D., Schuerch, F., Seger, M., Schreiner, K., Daeffler, R., Osmani, A., Bixel, D., Loiseleur, O., Cercus, J., Stettler, H., Schaer, K., Gamboni, R.: Large-scale synthesis of the anti-cancer marine natural product (+)-discodermolide. Part 4: preparation of fragment C7–24. Org. Proc. Res. Dev. 8, 113–121 (2004). (c) Mergott, D.J., Frank, S.A., Roush, W.R.: Total synthesis of (–)-spinosyn A. Proc. Natl. Acad. Sci. U. S. A. 101, 11955–11959 (2004). (d) Archambaud, S., Aphecetche-Julienne, K., Guingant, A.: A new total synthesis of (+)-Brefeldin C. Synlett 139–143 (2005). (e) Ehrlich, G., Kalesse, M.: Synthesis of the C13–C23 segment of tedanolide. Synlett 655–657, 2005. (f) Merschaert, A., Boquel, P., Van Hoeck, J.-P., Gorissen, H., Borghese, A., Bonnier, B., Mockel, A., Napora, F.: Novel approaches towards the LTD4/E4 antagonist, LY290154. Org. Process. Res. Dev. 10, 776–783 (2006). (g) Sharma, P.K., Kolchinski, A., Shea, H.A., Nair, J.J., Gou, Y., Romanczyk, L.J., Schmitz, H.H.: Scale-up syntheses of two naturally occurring procyanidins: (−)-epicatechin-(4β,8)-(+)-catechin and (−)-epicatechin-3-O-galloyl-(4β,8)-(−)-epicatechin-3-O-gallate. Org. Process. Res. Dev. 11, 422–430 (2007). (h) Armitage, M., Bret, G., Choudary, B.M., Kingswood, M., Loft, M., Moore, S., Smith, S., Urquhart, M.W.J.: Identification and development of an efficient route to SB-649915. Org. Process. Res. Dev. 16, 1626–1634 (2012)
Yamago, S., Miyazoe, H., Iida, K., Yoshida, J.-i.: Highly efficient and chemoselective reductive bis-silylation of quinones by silyltellurides. Org. Lett. 2, 3671–3673 (2000)
Ohkubo, K., Fujimoto, A., Fukuzumi, S.: Visible-light-induced oxygenation of benzene by the triplet excited state of 2,3-dichloro-5,6-dicyano-p-benzoquinone. J. Am. Chem. Soc. 135, 5368–5371 (2013)
Walsh, K., Sneddon, H.F., Moody, C.J.: Solar photochemical oxidations of benzylic and allylic alcohols using catalytic organo-oxidation with DDQ: application to lignin models. Org. Lett. 16, 5224–5227 (2014)
Mitchell, L.J., Moody, C.J.: Solar photochemical oxidation of alcohols using catalytic hydroquinone and copper nanoparticles under oxygen: oxidative cleavage of lignin models. J. Org. Chem. 79, 11091–11100 (2014)
Johansson, M., Purse, B.W., Terasaki, O., Bäckvall, J.-E.: Aerobic oxidations catalyzed by zeolite-encapsulated cobalt salophen. Adv. Synth. Catal. 350, 1807–1815 (2008)
Zaman, K.M., Yamamoto, S., Nishimura, N., Maruta, J., Fukuzumi, S.: Charge-transfer complexes acting as real intermediates in hydride transfer from Michler’s hydride to 2,3-dichloro-5,6-dicyano-p-benzoquinone via electron transfer. J. Am. Chem. Soc. 116, 12099–12100 (1994)
Yamamoto, S., Sakurai, T., Yingjin, L., Sueishi, Y.: Mechanism of hydride transfer reaction from 4-(dimethylamino)phenyl methane derivatives to 2,3-dichloro-5,6-dicyano-p-benzoquinone. Phys. Chem. Chem. Phys. 1, 833–837 (1999)
Fukuzumi, S., Ohkubo, K., Tokuda, Y., Suenobu, T.: Hydride transfer from 9-substituted 10-methyl-9,10-dihydroacridines to hydride acceptors via charge-transfer complexes and sequential electron−proton−electron transfer. A negative temperature dependence of the rates. J. Am. Chem. Soc. 122, 4286–4294 (2000)
Shunichi, F., Morifumi, F., Gen-etsu, M., Junzo, O.: Electron transfer vs. nucleophilic addition of ketene silyl acetals with halogenated p-benzoquinone derivatives. Chem. Lett. 22, 1451–1454 (1993)
Guo, X., Mayr, H.: Manifestation of polar reaction pathways of 2,3-dichloro-5,6-dicyano-p-benzoquinone. J. Am. Chem. Soc. 135, 12377–12387 (2013)
Nishinaga, A., Rindo, K., Matsuura, T.: A convenient synthesis of acyclic 1,n-diketones (n = 5–8) from 2-t-butylperoxycycloalkanones. Synthesis 1986, 1038–1041 (1986)
Gu, X., Zhang, W., Salomon, R.G.: Fragmentation of β-hydroxy hydroperoxides. J. Org. Chem. 77, 1554–1559 (2012)
For visible-light mediated C–C bond cleavage of 1,2-diols to carbonyls, see: (a) Ito, Y., Kunimoto, K., Miyachi, S., Kako, T.: Photocatalytic cleavage of 1,2-diols by a cofacially hindered water-soluble iron(III) porphyrin. Tetrahedron Lett. 32, 4007–4010 (1991). (b) Matsusaki, Y., Yamaguchi, T., Tada, N., Miura, T., Itoh, A.: Aerobic photooxidative cleavage of vicinal diols to carboxylic acids using 2-chloroanthraquinone. Synlett 23, 2059–2062 (2012). (c) Schwarz, J., König, B.: Visible-light mediated C–C bond cleavage of 1,2-diols to carbonyls by cerium-photocatalysis. Chem. Commun. 55, 486–488 (2019)
For aerobic, thermal manganese-catalyzed cleavage of 1,2-diols, see: (a) Escande, V., Lam, C.H., Coish, P., Anastas, P.T.: Heterogeneous sodium-manganese oxide catalyzed aerobic oxidative cleavage of 1,2-diols. Angew. Chem. Int. Ed. 56, 9561–9565 (2017). (b) Escande, V., Lam, C.H., Grison, C., Anastas, P.T.: EcoMnOx, a biosourced catalyst for selective aerobic oxidative cleavage of activated 1,2-diols. ACS Sustain. Chem. Eng. 5, 3214–3222 (2017)
For oxidative cleavage of 1,2-diols with DMSO as oxidant, see: (c) García, N., Rubio-Presa, R., García-García, P., Fernández-Rodríguez, M.A., Pedrosa, M.R., Arnáiz, F.J., Sanz, R.: A selective, efficient and environmentally friendly method for the oxidative cleavage of glycols. Green Chem. 18, 2335–2340 (2016)
For aerobic, thermal palladium-catalyzed cleavage of 1,2-diols, see: Wang, A., Jiang, H.: Palladium-catalyzed direct oxidation of alkenes with molecular oxygen: general and practical methods for the preparation of 1,2-diols, aldehydes, and ketones. J. Org. Chem. 75, 2321–2326 (2010)
For aerobic, thermal ruthenium-catalyzed cleavage of 1,2-diols, see: (a) Felthouse, T.R.: Catalytic oxidative cleavage of vicinal diols and related oxidations by ruthenium pyrochlore oxides: new catalysts for low-temperature oxidations with molecular oxygen. J. Am. Chem. Soc. 109, 7566–7568 (1987). (b) Takezawa, E., Sakaguchi, S., Ishii, Y.: Oxidative cleavage of vic-diols to aldehydes with dioxygen catalyzed by Ru(PPh3)3Cl2 on active carbon. Org. Lett. 1, 713–715 (1999)
For aerobic, thermal silver-catalyzed cleavage of 1,2-diols, see: Zhou, Z.-z., Liu, M., Lv, L., Li, C.-J.: Silver(I)-catalyzed widely applicable aerobic 1,2-diol oxidative cleavage. Angew. Chem. Int. Ed. 57, 2616–2620 (2018)
For aerobic, thermal vanadium-catalyzed cleavage of 1,2-diols, see: (a) Hanson, S.K., Baker, R.T., Gordon, J.C., Scott, B.L., Sutton, A.D., Thorn, D.L.: Aerobic oxidation of pinacol by vanadium(V) dipicolinate complexes: evidence for reduction to vanadium(III). J. Am. Chem. Soc. 131, 428–429 (2009). (b) Kirihara, M., Yoshida, K., Noguchi, T., Naito, S., Matsumoto, N., Ema, Y., Torii, M., Ishizuka, Y., Souta, I.: Effective cleavage of ditertiary glycols via vanadium(V)-catalyzed aerobic oxidation. Tetrahedron Lett. 51, 3619–3622 (2010). (c) Obara, N., Hirasawa, S., Tamura, M., Nakagawa, Y., Tomishige, K.: Oxidative cleavage of vicinal diols with the combination of platinum and vanadium catalysts and molecular oxygen. ChemCatChem 8, 1732–1738 (2016). (d) Amadio, E., González-Fabra, J., Carraro, D., Denis, W., Gjoka, B., Zonta, C., Bartik, K., Cavani, F., Solmi, S., Bo, C., Licini, G.: Efficient vanadium-catalyzed aerobic C−C bond oxidative cleavage of vicinal diols. Adv. Synth. Catal. 360, 3286–3296 (2018)
For early studies on vanadium-catalyzed cleavage of 1,2-diols, see: (a) Littler, J.S., Mallet, A.I., Waters, W.A.: 551. Oxidations of organic compounds with quinquevalent vanadium. Part IV. The oxidation of some α-glycols. J. Chem. Soc. 2761–2766 (1960). (b) Zviely, M., Goldman, A., Kirson, I., Glotter, E.: Selective cleavage of ditertiary glycols under mild conditions, with bisacetylacetonato-oxovanadium. J. Chem. Soc. Perkin Trans. 1, 229–231 (1986)
For aerobic cleavage of 1,2-diols using polyoxometalates (POMs), see: (a) Shimizu, M., Orita, H., Suzuki, K., Hayakawa, T., Hamakawa, S., Takehira, K.: Oxidative C–C bond cleavage of vic-diols with H2O2 catalyzed by heteropolyacids. J. Mol. Catal. A Chem. 114, 217–220 (1996). (b) Santacesaria, E., Sorrentino, A., Rainone, F., Di Serio, M., Speranza, F.: Oxidative cleavage of the double bond of monoenic fatty chains in two steps: a new promising route to azelaic acid and other industrial products. Ind. Eng. Chem. Res. 39, 2766–2771 (2000). (c) Khenkin, A.M., Neumann, R.: Aerobic oxidation of vicinal diols catalyzed by an Anderson-type polyoxometalate, [IMo6O24]5−. Adv. Synth. Catal. 344, 1017–1021 (2002)
For selected reviews on metal-catalyzed carbon–carbon bond cleavage, see: (a) Chen, F., Wang, T., Jiao, N.: Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev. 114, 8613–8661 (2014). (b) Marek, I., Masarwa, A., Delaye, P.-O., Leibeling, M.: Selective carbon–carbon Bond cleavage for the Stereoselective synthesis of acyclic systems. Angew. Chem. Int. Ed. 54, 414–429 (2015). (c) Souillart, L., Cramer, N.: Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9464 (2015). (d) Nairoukh, Z., Cormier, M., Marek, I.: Merging C–H and C–C bond cleavage in organic synthesis. Nat. Rev. Chem. 1, 0035 (2017). (e) Fumagalli, G., Stanton, S., Bower, J.F.: Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 117, 9404–9432 (2017)
For aerobic, thermal vanadium-catalyzed oxidation and cleavage of lignin model compounds, see: (a) Hanson, S.K., Baker, R.T., Gordon, J.C., Scott, B.L., Thorn, D.L. Aerobic oxidation of lignin models using a base metal vanadium catalyst. Inorg. Chem. 49, 5611–5618 (2010). (b) Hanson, S.K., Wu, R., Silks, L.A. C–C or C–O Bond cleavage in a phenolic lignin model compound: selectivity depends on vanadium catalyst. Angew. Chem. Int. Ed. 51, 3410–3413 (2012). (c) Zhang, G., Scott, B.L., Wu, R., Silks, L.A., Hanson, S.K. Aerobic oxidation reactions catalyzed by vanadium complexes of bis(phenolate) ligands. Inorg. Chem. 51, 7354–7361 (2012). For a review, see: (d) Hanson, S.K., Baker, R.T. Knocking on wood: base metal complexes as catalysts for selective oxidation of lignin models and extracts. Acc. Chem. Res. 48, 2037–2048 (2015)
For a review on vanadium-catalyzed aerobic carbon–carbon bond cleavage, see: Amadio, E., Di Lorenzo, R., Zonta, C., Licini, G.: Vanadium catalyzed aerobic carbon–carbon cleavage. Coord. Chem. Rev. 301–302, 147–162 (2015)
For reports on copper-mediated carbon−carbon bond oxidative cleavage of lignin models and native lignin, see: (a) Wang, M., Lu, J., Zhang, X., Li, L., Li, H., Luo, N., Wang, F.: Two-step, catalytic C–C bond oxidative cleavage process converts lignin models and extracts to aromatic acids. ACS Catal. 6, 6086–6090 (2016). (b) Ren, X., Wang, P., Han, X., Zhang, G., Gu, J., Ding, C., Zheng, X., Cao, F.: Depolymerization of lignin to aromatics by selectively oxidizing cleavage of C–C and C–O bonds using CuCl2/polybenzoxazine catalysts at room temperature. ACS Sustain. Chem. Eng. 5, 6548–6556 (2017). (c) Salonen, H.E.P., Mecke, C.P.A., Karjomaa, M.I., Joensuu, P.M., Koskinen, A.M.P.: Copper catalyzed alcohol oxidation and cleavage of β-O-4 lignin model systems: from development to mechanistic examination. ChemistrySelect 3, 12446–12454 (2018)
For a review on catalytic cleavage of carbon–carbon bonds in lignin model compounds, see: Guadix-Montero, S., Sankar, M.: Review on catalytic cleavage of C–C inter-unit linkages in lignin model compounds: towards lignin depolymerisation. Top. Catal. 61, 183–198 (2018)
Gazi, S., Hung Ng, W.K., Ganguly, R., Putra Moeljadi, A.M., Hirao, H., Soo, H.S.: Selective photocatalytic C–C bond cleavage under ambient conditions with earth abundant vanadium complexes. Chem. Sci. 6, 7130–7142 (2015)
Gazi, S., Đokić, M., Putra Moeljadi, A.M., Ganguly, R., Hirao, H., Soo, H.S.: Kinetics and DFT studies of photoredox carbon−carbon bond cleavage reactions by molecular vanadium catalysts under ambient conditions. ACS Catal. 7, 4682–4691 (2017)
Dicosimo, R., Szabo, H.-C.: Oxidation of lignin model compounds using single-electron-transfer catalysts. J. Org. Chem. 53, 1673–1679 (1988)
Cho, D.W., Parthasarathi, R., Pimentel, A.S., Maestas, G.D., Park, H.J., Yoon, U.C., Mariano-Dunaway, D., Gnanakaran, S., Langan, P., Mariano, P.S.: Nature and kinetic analysis of carbon–carbon bond fragmentation reactions of cation radicals derived from SET-oxidation of lignin model compounds. J. Org. Chem. 75, 6549–6562 (2010)
Cho, D.W., Latham, J.A., Park, H.J., Yoon, U.C., Langan, P., Dunaway-Mariano, D., Mariano, P.S.: Regioselectivity of enzymatic and photochemical single electron transfer promoted carbon–carbon bond fragmentation reactions of tetrameric lignin model compounds. J. Org. Chem. 76, 2840–2852 (2011)
Lim, S.H., Nahm, K., Ra, C.S., Cho, D.W., Yoon, U.C., Latham, J.A., Dunaway-Mariano, D., Mariano, P.S.: Effects of alkoxy groups on arene rings of lignin β-O-4 model compounds on the efficiencies of single electron transfer-promoted photochemical and enzymatic C–C bond cleavage. J. Org. Chem., 78, 9431–9443 (2013)
Lim, S.H., Lee, W.S., Kim, Y.-I., Sohn, Y., Cho, D.W., Kim, C., Kim, E., Latham, J.A., Dunaway-Mariano, D., Mariano, P.S.: Photochemical and enzymatic SET promoted C–C bond cleavage reactions of lignin β-1 model compounds containing varying number of methoxy substituents on their arene rings. Tetrahedron. 71, 4236–4247 (2015)
Narayanam, J.M.R., Tucker, J.W., Stephenson, C.R.J.: Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 131, 8756–8757 (2009)
Hasegaway, E., Takizawa, S., Seida, T., Yamaguchi, A., Yamaguchi, N., Chiba, N., Takahashi, T., Ikeda, H., Akiyama, K.: Photoinduced electron-transfer systems consisting of electron-donating pyrenes or anthracenes and benzimidazolines for reductive transformation of carbonyl compounds. Tetrahedron. 62, 6581–6588 (2006)
Larraufie, M.-H., Pellet, R., Fensterbank, L., Goddard, J.-P., Lacôte, E., Malacria, M., Ollivier, C.: Visible-light-induced photoreductive generation of radicals from epoxides and aziridines. Angew. Chem. Int. Ed. 50, 4463–4466 (2011)
Kim, S., Chmely, S.C., Nimlos, M.R., Bomble, Y.J., Foust, T.D., Paton, R.S., Beckham, G.T.: Computational study of bond dissociation enthalpies for a large range of native and modified lignins. J. Phys. Chem. Lett. 2, 2846–2852 (2011)
Nguyen, J.D., Matsuura, B.S., Stephenson, C.R.J.: A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc. 136, 1218–1221 (2014)
For a discussion on the use of an initial oxidation step as an approach for minimizing lignin fragment condensations, see: Zhang, C., Wang, F.: Catalytic lignin depolymerization to aromatic chemicals. Acc. Chem. Res. 53, 470–484 (2020)
For selected reports on selective oxidation of lignin models and lignin, see: (a) Rahimi, A., Azarpira, A., Kim, H., Ralph, J., Stahl, S.S.: Chemoselective metal-free aerobic alcohol oxidation in lignin. J. Am. Chem. Soc. 135, 6415–6418 (2013). (b) Lancefield, C.S., Ojo, O.S., Tran, F., Westwood, N.J.: Isolation of functionalized phenolic monomers through selective oxidation and C–O bond cleavage of the β-O-4 linkages in lignin. Angew. Chem. Int. Ed. 54, 258–262 (2015). (c) Wang, M., Lu, J., Zhang, X., Li, L., Li, H., Luo, N., Wang, F.: Two-step, catalytic C–C bond oxidative cleavage process converts lignin models and extracts to aromatic acids. ACS Catal. 6, 6086–6090 (2016). (d) Dabral, S., Hernández, J.G., Kamer, P.C.J., Bolm, C.: Organocatalytic chemoselective primary alcohol oxidation and subsequent cleavage of lignin model compounds and lignin. ChemSusChem 10, 2707–2713 (2017). (e) Zhang, C., Li, H., Lu, J., Zhang, X., MacArthur, K.E., Heggen, M., Wang, F.: Promoting lignin depolymerization and restraining the condensation via an oxidation−hydrogenation strategy. ACS Catal. 7, 3419–3429 (2017). (f) Panovic, I., Lancefield, C.S., Phillips, D., Gronnow, M.J., Westwood, N.J.: Selective primary alcohol oxidation of lignin streams from butanol-pretreated agricultural waste biomass. ChemSusChem 12, 542–548 (2019). (g) Lan, W., Behaghel de Bueren, J., Luterbacher, J. S.: Highly selective oxidation and depolymerization of α,γ-diol-protected lignin. Angew. Chem. Int. Ed. 58, 2649–2654 (2019). (h) Rafiee, M., Alherech, M., Karlen, S.D., Stahl, S.S.: Electrochemical aminoxyl-mediated oxidation of primary alcohols in lignin to carboxylic acids: polymer modification and depolymerization. J. Am. Chem. Soc. 141, 15266–15276 (2019). (i) Zhang, Z., Zijlstra, D.S., Lahive, C.W., Deuss, P.J.: Combined lignin defunctionalisation and synthesis gas formation by acceptorless dehydrogenative decarbonylation. Green Chem. 22, 3791–3801 (2020)
For selected reviews on flow photochemistry, see: (a) Knowles, J.P., Elliott, L.D., Booker-Milburn, K.I.: Flow photochemistry: old light through new windows. Beilstein J. Org. Chem. 8, 2025–2052 (2012). (b) Garlets, Z.J., Nguyen, J.D., Stephenson, C.R.J.: The development of visible-light Photoredox catalysis in flow. Isr. J. Chem. 54, 351–360 (2014). (c) Su, Y., Straathof, N. J. W., Hessel, V., Noël, T.: Photochemical transformations accelerated in continuous-flow reactors: basic concepts and applications. Chem. Eur. J. 20, 10562–10589 (2014). (d) Cambié, D., Bottecchia, C., Straathof, N.J.W., Hessel, V., Noël, T.: Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 116, 10276–10341 (2016). (e) Plutschack, M.B., Pieber, B., Gilmore, K., Seeberger, P.H.: The Hitchhiker’s guide to flow chemistry. Chem. Rev. 117, 11796–11893 (2017). (f) Politano, F., Oksdath-Mansilla, G.: Light on the horizon: current research and future perspectives in flow photochemistry. Org. Process. Res. Dev. 22, 1045–1062 (2018)
DiRocco, D.A., Dystra, K., Krska, S., Vachal, P., Conway, D.V., Tudge, M.: Late-stage functionalization of biologically active heterocycles through photoredox catalysis. Angew. Chem. Int. Ed. 53, 4802–4806 (2014)
Beatty, J.W., Douglas, J.J., Miller, R., McAtee, R.C., Cole, K.P., Stephenson, C.R.J.: Photochemical perfluoroalkylation with pyridine N-oxides: mechanistic insights and performance on a kilogram scale. Chem. 1, 456–472 (2016)
Douglas, J.J., Sevrin, M.J., Cole, K.P., Stephenson, C.R.J.: Preparative scale demonstration and mechanistic investigation of a visible light-mediated radical smiles rearrangement. Org. Process. Res. Dev. 20, 1148–1155 (2016)
Yayla, H.G., Peng, F., Mangion, I.K., McLaughlin, M., Campeau, L.-C., Davies, I.W., DiRocco, D.A., Knowles, R.R.: Discovery and mechanistic study of a photocatalytic indoline dehydrogenation for the synthesis of elbasvir. Chem. Sci. 7, 2066–2073 (2016)
Le, C., Wismer, M.K., Shi, Z.-C., Zhang, R., Conway, D.V., Li, G., Vachal, P., Davies, I.W., MacMillan, D.W.C.: A general small-scale reactor to enable standardization and acceleration of photocatalytic reactions. ACS Cent. Sci. 3, 647–653 (2017)
Harper, K.C., Moschetta, E.G., Bordawekar, S.V., Wittenberger, S.J.: A laser driven flow chemistry platform for scaling photochemical reactions with visible light. ACS Cent. Sci. 5, 109–115 (2019)
Kärkäs, M.D., Bosque, I., Matsuura, B.S., Stephenson, C.R.J.: Photocatalytic oxidation of lignin model systems by merging visible-light photoredox and palladium catalysis. Org. Lett. 18, 5166–5169 (2016)
Magallanes, G., Kärkäs, M.D., Bosque, I., Lee, S., Maldonado, S., Stephenson, C.R.J.: Selective C−O bond cleavage of lignin systems and polymers enabled by sequential palladium-catalyzed aerobic oxidation and visible-light photoredox catalysis. ACS Catal. 9, 2252–2260 (2019)
For leading reviews on organic electrosynthesis, see: (a) Francke, R., Little, R.D.: Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014). (b) Yan, M., Kawamata, Y., Baran, P.S.: Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017). (c) Moeller, K.D.: Using physical organic chemistry to shape the course of electrochemical reactions. Chem. Rev. 118, 4817–4833 (2018). (d) Wiebe, A., Gieshoff, T., Möhle, S., Rodrigo, E., Zirbes, M., Waldvogel, S.R.: Electrifying organic synthesis. Angew. Chem. Int. Ed. 57, 5594–5619 (2018). (e) Möhle, S., Zirbes, M., Rodrigo, E., Gieshoff, T., Wiebe, A., Waldvogel, S.R.: Modern electrochemical aspects for the synthesis of value-added organic products. Angew. Chem. Int. Ed. 57, 6018–6041 (2018). (f) Kärkäs, M.D.: Electrochemical strategies for C–H functionalization and C–N bond formation. Chem. Soc. Rev. 47, 5786–5865 (2018). (h) Sauermann, N., Meyer, T.H., Qiu, Y., Ackermann, L.: Electrocatalytic C–H activation. ACS Catal. 8, 7086–7103 (2018)
Bosque, I., Magallanes, G., Rigoulet, M., Kärkäs, M.D., Stephenson, C.R.J.: Redox catalysis facilitates lignin depolymerization. ACS Cent. Sci. 3, 621–628 (2017)
Luo, J., Zhang, J.: Aerobic oxidation of olefins and lignin model compounds using photogenerated phthalimide-N-oxyl radical. J. Org. Chem. 81, 9131–9137 (2016)
Luo, J., Zhang, X., Lu, J., Zhang, J.: Fine tuning the redox potentials of carbazolic porous organic frameworks for visible-light photoredox catalytic degradation of lignin β-O-4 models. ACS Catal. 7, 5062–5070 (2017)
Hao, Z., Li, S., Sun, J., Li, S., Zhang, F.: Efficient visible-light-driven depolymerization of oxidized lignin to aromatics catalyzed by an iridium complex immobilized on mesocellular silica foams. Appl. Catal. B Environ. 237, 366–372 (2018)
Tay, N.E.S., Nicewicz, D.A.: Cation radical accelerated nucleophilic aromatic substitution via organic photoredox catalysis. J. Am. Chem. Soc. 139, 16100–16104 (2017)
Roth, H.G., Romero, N.A., Nicewicz, D.A.: Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett. 27, 714–723 (2016)
Romero, N.A., Margrey, K.A., Tay, N.E., Nicewicz, D.A.: Site-selective arene C–H amination via photoredox catalysis. Science. 349, 1326–1330 (2015)
McManus, J.B., Nicewicz, D.A.: Direct C–H cyanation of arenes via organic photoredox catalysis. J. Am. Chem. Soc. 139, 2880–2883 (2017)
Li, H., Bunrit, A., Lu, J., Gao, Z., Luo, N., Liu, H., Wang, F.: Photocatalytic cleavage of aryl ether in modified lignin to non-phenolic aromatics. ACS Catal. 9, 8843–8851 (2019)
Zhou, W., Nakahashi, J., Miura, T., Murakami, M.: Light/copper relay for aerobic fragmentation of lignin model compounds. Asian J. Org. Chem. 7, 2431–2434 (2018)
Sedai, B., Diaz-Urrutia, C., Baker, R.T., Wu, R., Silks, L.A., Hanson, S.K.: Comparison of copper and vanadium homogeneous catalysts for aerobic oxidation of lignin models. ACS Catal. 1, 794–804 (2011)
Sedai, B., Diaz-Urrutia, C., Baker, R.T., Wu, R., Silks, L.A., Hanson, S.K.: Aerobic oxidation of β-1 lignin model compounds with copper and oxovanadium catalysts. ACS Catal. 3, 3111–3122 (2013)
Yayla, H.G., Wang, H., Tarantino, K.T., Orbe, H.S., Knowles, R.R.: Catalytic ring-opening of cyclic alcohols enabled by PCET activation of strong O–H bonds. J. Am. Chem. Soc., 138, 10794–10797 (2016)
Ota, E., Wang, H., Frye, N.L., Knowles, R.R.: A redox strategy for light-driven, out-of-equilibrium isomerizations and application to catalytic C–C bond cleavage reactions. J. Am. Chem. Soc. 141, 1457–1462 (2019)
For a mechanistic study on multisite proton-coupled electron transfer activation of aryl ketones, see: Qiu, G., Knowles, R.R.: Rate-driving force relationships in the multisite-PCET activation of ketones. J. Am. Chem. Soc. 141, 2721–2730 (2019)
Wang, Y., Liu, Y., He, J., Zhang, Y.: Redox-neutral photocatalytic strategy for selective C–C bond cleavage of lignin and lignin models via PCET process. Sci. Bull. 64, 1658–1666 (2019)
For a related approach using cerium-mediated photocatalysis, see: Wang, Y., He, J., Zhang, Y.: CeCl3-promoted simultaneous photocatalytic cleavage and amination of Cα–Cβ bond in lignin model compounds and native lignin. CCS Chem. 2, 107–117 (2020)
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Magallanes, G., Kärkäs, M.D., Stephenson, C.R.J. (2022). Depolymerization of Lignin by Homogeneous Photocatalysis. In: Bahnemann, D., Patrocinio, A.O.T. (eds) Springer Handbook of Inorganic Photochemistry. Springer Handbooks. Springer, Cham. https://doi.org/10.1007/978-3-030-63713-2_52
Download citation
DOI: https://doi.org/10.1007/978-3-030-63713-2_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63712-5
Online ISBN: 978-3-030-63713-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)