Abstract
With modern radiotherapy techniques, re-irradiation is a potentially curative treatment approach. However, it carries still a serious risk of severe radiation morbidity including treatment-related death. High-level evidence for an adequate treatment of these patients is still lacking since the majority of trials are retrospective. Thus, inherent biases like unbalanced patients’ characteristics in terms of the relapse intervals, sites of recurrence, and inhomogeneous re-irradiation volumes, total tumor doses, and fractionation are by definition competing risks that will flaw the validity of these studies. Hence, future well-designed prospective randomized trials are still urgently needed.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Radiotherapy (RT), alone or in combination with surgery and chemotherapy, is a mainstay of curative treatment of patients with head and neck cancer (HNC). Despite advances in treatment and intensification of regimens, e.g. chemoradiation (CRT) [1] or alternative fractionated RT [2, 3], locoregional recurrence as the predominant pattern of failure occurs in 15–50% of patients and represents the most common cause of death in this patient population [4,5,6,7]. Most recurrences emerge during the first 2 years after initial RT and 80% occur in-field of formerly irradiated volumes [8]. Furthermore, a second primary HNC in the previously irradiated volume is frequently encountered [9]. It may arise from field cancerization, radiation-induced changes, or de novo from past or continued tobacco or alcohol abuse. Whenever feasible, salvage surgery is the treatment of choice. However many patients are not surgical candidates due to comorbidities, disease progression to an unresectable stage, or patient preferences.
Re-irradiation (Re-RT) is a potentially curative treatment option but represents a challenging problem and carries a poor prognosis. Re-RT with conventional radiation techniques (2D or 3D conformal RT) carries a serious risk of treatment-related toxicities, including treatment-related deaths [10]. With conventional RT-techniques only, small gains of Re-RT compared with chemotherapy alone could be seen in oncological outcome which did not justify the high-grade toxicity experienced by the patients [11, 12]. Modern radiation techniques like intensity-modulated radiotherapy (IMRT), stereotactic body radiotherapy (SBRT), and proton therapy (PT) have shown improved disease control compared with conventional 2D or 3D Re-RT by more precisely delivering high radiation doses to target volumes while reducing toxicities. These improvements open up the possibility to ask the question again which strategy of Re-RT in the setting of HNC is the most successful. Many studies on Re-RT with modern radiation techniques have already been conducted. However, interpretation of their results is ambiguous, especially due to the low number of patients per study, a variety of treatment regimens used, an inherent heterogeneity of patient characteristics, and possible biases resulting from mostly retrospective evaluations. This chapter will focus on patient selection strategies, choice of an adequate treatment regimen, expected oncological outcome, and toxicities after Re-RT.
When to re-Irradiate?
With a careful patient selection, Re-RT can achieve a longer progression-free survival (PFS) and disease-free survival (DFS); however, severe acute and late side effects must be taken into account. Therefore, Re-RT's adequate patient selection criteria are crucial to avoid unnecessary toxicities, and a further reduction of quality of life (QoL) in patients whose life expectancy already is limited. Several prognostic factors for survival after HNC Re-RT have been reported. An appropriate algorithm for treatment selection needs to consider factors related to the disease, comorbidities, and organ dysfunction. A simplified decision tree can be found in Fig. 17.1. Ideally, Re-RT should always be based on a therapeutic decision of an interdisciplinary tumor board. Comprehensive informed patient consent regarding toxicities and expected benefits from the treatment are necessary prerequisites for joint decision-making. Ideally, patients should be included in prospective randomized clinical trials to generate better models on individually predictive factors, which would allow a more precise treatment selection in this extremely vulnerable patient population.
Algorithm for treatment selection for patients with HNC recurrence or second primary HNC in a previously irradiated location. Modified from Strojan et al. [13]. Abbreviations: RT radiotherapy, CTX chemotherapy, Re-RT re-irradiation, ECE+ extracapsular extension, R1 microscopic resection margin, R2 macroscopic residual tumor
Previous Toxicity and Patient-Related Considerations
Limiting toxicity and maintaining organ function should be the major priority when considering Re-RT. There is no consensus concerning the cumulative dose of organs at risk (OARs) when performing Re-RT. Due to a known heterogeneity of radiosensitivity in patients [14], it is important to consider the treatment-related toxicity of the initial RT. Patients with higher-grade toxicities from initial RT such as osteoradionecrosis, severe fibrosis, or dysphagia should be excluded. Tanvetyanon et al. found that pre-existing organ dysfunction and comorbidities belong to the most important factors of long-term outcome. A nomogram was created on the basis of these findings to predict the 24 months survival probability after Re-RT [15].
Treatment Volume and Recurrent Stage
Various studies have found that patients with smaller treatment volumes have higher locoregional control (LRC) and overall survival (OS) rates after Re-RT with thresholds of gross tumor volume (GTV) <15 cm3 [16] and < 25 cm3 [17,18,19] and of planning target volume (PTV) <27 cm3 [20] and ≤ 40 cm3 [21]. In a retrospective study with 91 patients receiving Re-RT for locally recurrent nasopharyngeal carcinoma (NPC), the 3-year local failure-free survival rates for rT1, rT2, and rT3 were 64%, 61.5%, and 18.4% [22], respectively. Additionally, it has been reported that a GTV >25 cm3 was predictive for acute toxicities in a series of SBRT treatments [18, 19]. With IMRT, a clinical target volume (CTV) ≥40 cm3 was associated with increased late toxicity [23, 24]. Consequently, Re-RT for patients with bulky tumors in a curative approach should only be offered with caution [25].
Time Interval since Initial Radiotherapy
Several studies suggest that the time interval since initial RT is prognostic for OS. In a phase II trial (RTOG 9610), patients (n = 86) with recurrent HNC or second primary HNC arising in a previously iradiated field were enrolled to receive CRT with 1.5 Gy twice-daily and 4 cycles of 5-fluorouracil (5-FU) and hydroxyurea. Patients who received Re-RT less than 1 year from initial RT had a significantly worse OS than patients with an interval of more than 1 year (median OS 7.7 vs 9.8 months, p = .033) [12]. More studies support the finding that the time interval since initial RT is an independent factor for OS [26, 27]. While no minimum time interval between Re-RT and the previous RT is established, most trials require intervals of at least 6 months with a longer interval preferred. A study by Chen et al. found a higher risk of toxicity in patients receiving Re-RT with a shorter time interval than 1 year [25].
Anatomical Site
Outcomes also correlate with the site of recurrence with nasopharyngeal and laryngeal cancer revealing a better prognosis [23, 28,29,30,31,32]. Generally, nasopharyngeal tumors have a higher radiosensitivity which could be also an explanation for its favorable prognosis with Re-RT. For early-stage rT1/rT2 cases, brachytherapy is effective to achieve a 5-year local control rates of 85% and OS of 61.3% [33]. In a retrospective series of 90 patients treated with SBRT with a median dose of 18 Gy in 3 fractions or 48 Gy in 6 fractions, a 3-year local failure-free survival of 89.4% could be achieved. Also, for selected patients with laryngeal carcinoma good clinical outcomes have been described. Patients with recurrent early stage I and II laryngeal carcinomas were treated with Re-RT and a cumulative dose ranging from 60 to 70 Gy. Five-year local control and OS rates of 60% and 93%, respectively, were observed while the majority of patients had a functional larynx [31].
Second Primary vs Recurrent Tumors
It is plausible to assume that second primary cancers in a previously radiated volume could respond better to Re-RT compared with recurrent HNC due to the inherent radioresistence of recurrent tumor cells. Several studies support this assumption. In RTOG 96–10, patients with a second primary had a 1-year OS rate of 54% and median survival time of 19.8 months compared with 38% and 7.7 months for patients with recurrent HNC [34]. In another study conducted by Stevens et al., 100 patients treated with Re-RT alone had a 5-year LRC of 60% and 37% OS for secondary primaries compared with 27% and 17% for patients with recurrent HNC [35].
Re-Irradiation after Salvage Surgery
A multicenter phase III randomized controlled trial (RCT) compared salvage surgery and re-chemoradiation (Re-CRT) with salvage surgery only [36]. Patients (n = 130) were recruited at 16 French and Belgian sites. Eighty-four percent of the patients underwent lymph node dissection. Higher risk factors were evaluated by histopathological examination, namely positive or close margins, extracapsular extension, or metastases in more than one lymph node. In the Re-CRT-arm, patients received 6 cycles of 2 Gy fractions for 5 days (60 Gy total) concomitant with hydroxyurea and 5-FU over the course of 11 weeks. A significant improvement in LRC (HR 4.51, p < 0.001) and DFS (HR 1.68, p < 0.01) in favor of the Re-CRTarm were observed (Fig. 17.2). However, no difference in OS could be noticed which might relate to treatment-associated death, distant metastases, and second primary tumors in the Re-CRT group. Also, severe toxicity (grade 3 and 4) at 2-years could have contributed in the Re-CRT arm with 39% vs 10% in the surgery alone arm (p = 0.06).
Kaplan-Meier pots for LCR in patients with recurrent HNC treated with postoperative Re-CRT. LRC was significantly improved in the Re-CRTarm vs salvage surgery alone (HR 4.51, p < 0.001) [36]
Take Home Message for when to re-Irradiate?
-
Careful patient selection for Re-RT remains paramount.
-
Appropriate patient selection criteria comprise factors related to the patient and disease.
-
Favorable factors are no comorbidities and adequate organ functions, small tumor volumes <40cm3, time interval from initial RT to Re-RT (>6 mos.) and nasopharyngeal or laryngeal tumors.
-
Decision for or against Re-RT should be based on the consensus of an interdisciplinary tumor board.
-
A comprehensive informed consent of patients concerning the benefits and risks of the treatment is a key issue for joint decision-making.
How to re-Irradiate?
Re-Irradiation Dose
Generally, there is no consensus on the radiation dose for target volume, a particular fractionation scheme, or allowed cumulative doses for OARs. Recurrent HNC after initial RT suggests the presence of radiation-resistant tumor cells which implies the need for a high dose Re-RT. This might be in particular true for patients who received CRT as initial treatment. Indeed, several studies have reported a dose-response relationship for improved LRC [15, 17, 23, 37]. In a study conducted by Salama et al., patients with a total dose of ≥58 Gy had a 3-year OS of 30% compared with 6% for patients receiving less than 58 Gy [10]. Other investigators use a slightly higher target dose of 60 Gy as found in several Re-RT protocols [38, 39]. In an SBRT study with 85 patients, local control (LC) was significantly higher in patients receiving ≥35 Gy compared with a lower dose (p = 0.014) [40]. Since all of these reports are retrospective, it is important to consider possible selection biases like patients with better performance status and smaller tumor volumes might have received higher radiation doses.
Re-Irradiation Volume
In the setting of Re-RT for local tumor recurrence, there is no debate about the need for a maximally tolerable total dose to the macroscopically recurrent tumor. However, elective irradiation to the neck is controversially discussed [39]. It can be argued that in most cases the elective neck did receive a lower total dose than the target volume of the primary during initial RT and could consequently tolerate an additional elective irradiation series, but it is evident that the hazards of toxicities increase with the size of the treatment volume. Moreover, in a multi-institutional retrospective analysis comprising 505 patients, elective nodal irradiation was not associated with an improved locoregional failure or OS but with increased risks of acute toxicities [41]. Based on the current study situation and opposed to RT in the primary setting, radiation of elective nodal volumes cannot be recommended.
Multiple studies have reported that the most common pattern of failure is local [23, 42, 43]. From recent studies with image-guided radiotherapy (IGRT) and computer-assisted RT planning, the clinical target volume (CTV) should include the gross tumor volume (GTV) or tumor bed plus a minimal safety margin [20, 23, 26, 44, 45] (Table 17.1). Target delineation based on computed tomography (CT) or positron emission tomography (PET)-CT scans, modern immobilization, and radiation techniques including IGRT, allow for a more precise Re-RT with smaller margins accounting for microscopic disease and positioning uncertainties.
Concurrent Systemic Therapy
The role of concurrent systemic therapy is not clearly defined because prospective randomized studies with a head to head comparison of Re-CRT with Re-RT alone are still lacking. Concurrent systemic therapies can be beneficial in terms of radiosensitization and harmful in terms of increasing toxicities [46], two factors that have to be carefully balanced in the setting of Re-RT. In a series of IMRT studies, Re-CRT has been administered at least to a part of the study population [11, 23, 42,43,44,45, 47,48,49,50]. Takiar et al. showed an improved LRC for patients receiving platinum-based Re-CRT, particularly when Re-RT was given adjuvantly [23]. There have also been studies reporting adverse outcomes with Re-CRT, although the findings might be biased by patient selection with advanced tumor stages and higher risk features [48, 49]. In a prospective phase II trial conducted by Tao et al., 53 patients were randomized after salvage surgery to receive either split course 60 Gy in 11 weeks with concomitant 5-FU/hydroxyurea or 60 Gy in 5 weeks with 1.2 Gy twice daily and cetuximab, which was found to be tolerable without significant acute toxicity [51].
The still dismal prognosis in pre-irradiated locally recurrent HNC with radiotherapy alone is a challenge and should lead to a combination of irradiation with appealing new drugs like the immune checkpoint inhibitors (CPIs). While the early experience with SBRT was gained without the addition of systemic therapies, recent studies have proved the safety of concurrent cetuximab with this approach [18, 52]. Results on CPIs in the setting of recurrent and metastatic HNC targeting cytotoxic lymphocyte antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1), and programmed cell death ligand-1 (PD-L1) have recently changed treatment paradigms and might have the potential to play a role in the Re-RT setting as well. There are currently several studies underway investigating this topic, e.g. the RTOG KEYSTROKE randomized phase II trial investigating the addition of Pembrolizumab to SBRT in patients with unresectable recurrent or second primary HNC.
Take Home Message for how to re-Irradiate?
-
Higher re-irradiation doses seem to be associated with better local tumor control.
-
Techniques for reduction of target volumes should be used, since larger treatment volumes are associated with increased toxicities.
-
After Re-RT, the predominant pattern of failure is in-field, therefore elective nodal irradiation is not recommended.
-
Concurrent systemic therapy should be offered to selected patients.
Radiation Techniques
Intensity-Modulated Radiotherapy (IMRT)
IMRT is a form of a radiation technique that uses multiple angled radiation fields or treatment arcs and intensity modulation to generate highly complex dose distributions. This enables to irradiate the target volume with conformality at higher doses while allowing for a more precise sparing of OARs. There is also the possibility to treat target volumes with an inhomogeneous dose. This technique is called “simultaneous integrated boost” which can deliver different dose levels to multiple target volumes. IMRT has already demonstrated its benefits in reducing toxicity for adjacent healthy tissue in the primary disease of HNCs [53]. Especially with Re-RT it is crucial to minimize the radiation exposure and cumulative dose of previously irradiated healthy tissues to reduce the risk of high grade toxicities. Likewise, it is important to deliver a high, tumoricidal dose to the target volume. Several studies have been conducted to evaluate the efficacy of IMRT for Re-RT of HNCs or second primary HNCs (Table 17.1). Most of these studies are retrospective with exception of the phase II trial RTOG 99–11 [11] and a prospective single institution registry trial [20]. All studies vary widely by different RT treatment regimens regarding the total dose and fractionation schedule, the application of concurrent chemotherapy, and the patient populations.
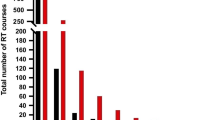
One of the first larger studies on the topic of Re-RT and IMRT that could show an improved oncological outcome was conducted by Lee at al [28]. Patients (n = 105) with recurrent HNC underwent Re-RT with 74 patients receiving IMRT and 31 patients 3D-conformal RT with a median dose of 59.4 Gy. An improved 2-year LRC of 52% could be observed with IMRT compared with 20% for 3D conformal RT (p < 0.001) (Fig. 17.3). A recurrence at the nasopharynx was associated with an improved LRC. Median OS was 15 months with a 2-year OS rate of 37%. Severe grade 3 and 4 late toxicities were reported in 15% of cases with a median onset of 6 months after Re-RT. Predictors of superior OS were non-squamous cell carcinoma (SCC) histology, recurrence at the nasopharynx site, and a Re-RT dose of ≥50 Gy. A retrospective study with a larger number of patients (n = 206) has been reported by Takiar et al. [23]. Patients were treated with IMRT and doses of 66 Gy in the definitive and 60 Gy in the adjuvant setting, and factors were correlated with oncological outcome. The 2-year OS and LRC rates were 57% and 65% respectively. SCC was associated with a worse prognosis compared with a non-SCC histology. Nasopharynx site and a 70 Gy Re-RT dose were associated with an improved outcome. Grade 3 toxicities and higher were reported in 32% after 2 years and 48% at 5 years and were associated with larger treatment volumes (>50 cm3). No grade ≥ 3 toxicities were observed for treatment volumes <25 cm3. Similar 2-year OS and LRC rates of 50% and 60% were reported by another IMRT study without grading of toxicities [49]. Duprez et al. reported the worst outcomes of IMRT studies, with 2-year OS and LCR of 32% and 48%, respectively [26]. Twenty percent of the patients developed grade 4 or higher toxicities. In a subset of patients, IMRT can offer a durable local control, however, severe late toxicities are not uncommon and treatment related-deaths can be observed in up to 11% of the cases.
Kaplan-Meier plots for LCR of patients with recurrent HNCs treated with either IMRT or non-IMRT (3D-conformal RT). LRC was significantly improved in the IMRT-arm vs the Non-IMRT-arm (2-year LRC 52% vs 20%, p < 0.001) [28]
Stereotactic Body Radiotherapy (SBRT)
SBRT is a highly precise RT which delivers hypofractionated doses of radiation to the tumor volume in a limited number of fractions. SBRT has already become the standard for several cancer treatments, e.g. brain metastases, early-stage lung cancer, or prostate cancer. Fewer fractions result in shorter overall treatment time from 5–7 weeks for standard IMRT regimes to 1 day to 2 weeks for SBRT. Additionally, there might be biological advantages to the ablative doses delivered, since other mechanisms of cell kill are activated than with lower doses used in conventionally fractionated RT. It could also be shown that shorter treatment times can result in better treatment outcomes, probably by overcoming the well-known repopulation effect found in HNC [56]. Sublethal damage repair, one mechanism of healthy tissue tolerance for conventionally fractionated RT, is lacking for SBRT. Therefore, another strategy with other definitions of precision and conformality of dose distribution has to be implemented to prevent toxicities.
There are two prospective studies supporting SBRT as Re-RT for recurrent HNC [52, 57]. The study of Comet et al. comprised 40 patients who received SBRT with a dose of 36 Gy in 6 fractions with a treatment interval of 11–12 days [52]. The authors reported a median OS of 13.6 months with a 79% response rate and grade 3 or higher toxicities in only 10% of the patients. Lartigau et al. conducted a multi-institutional phase II study with 56 patients and the same SBRT dose regimen but with the addition of cetuximab [57]. The authors reported a median OS of 11.8 months, 1-year OS of 48%, and a median PFS of 7.1 months. Treatment-related toxicities grade 3 or higher were observed in 32% of patients and one death from hemorrhage occurred.
Most other studies on the topic of SBRT in the setting of recurrent HNC published in the last couple of years have been retrospective. The study by Vargo et al. [55] is a pivotal multi-institutional study comprising a larger number of IMRT and SBRT cases with the aim to identify prognostic factors in both treatment modalities. The study found an improved OS associated with IMRT vs SBRT in the unadjusted model with a 2-year OS of 35.4% for IMRT and 16.3% for SBRT (p < 0.001). However, multivariable analysis accounting for other known prognostic factors did not show any significant difference between IMRT vs SBRT.
SBRT should be applied with caution if recurrences are located nearby critical organs like neurological structures or the carotid artery. One must be aware that normal tissues located partially inside or very close to the target volume receive the same ablative doses as the tumor itself potentially leading to impaired damage repair and consequential late damage [17]. Roman et al. developed a treatment selection algorithm for IMRT and SBRT based on the tumor location and ability of the patient to be treated with CTx [58]. A study by Yazici et al. [59] recommended using IMRT instead of SBRT if the maximum carotid artery dose exceeds 34 Gy if or more than 180° of the carotid artery is invaded. The same author also describes a reduced risk of severe toxicity by utilizing an every-other-day radiation protocol (Table 17.2).
Proton Therapy
Proton therapy (PT) might lead to additional benefits for a selected group of patients with recurrent HNC. The profile of energy deposition has potential dosimetric advantages to spare OARs since protons release most of their energy in the characteristic “Bragg peak” at the end of the rays. Beyond this peak, a steep dose fall-off occurs which can be exploited for precise OAR sparing. When considering one field, the PT beam's entry path receives a lower integral dose than with photons which further facilitates an improved OAR sparing. With PT, two different treatment techniques are used: (1) passive scattering proton therapy (PSPT) which uses scattering devices to broaden the proton beam and a range-modulation device to create a spread-out Bragg peak, and (2) intensity-modulated proton therapy (IMPT) taking advantage of bundles of scanning beams for further improvements of dose conformality compared with PSPT. PT Re-RT schemes for recurrent HNC have the potential of a substantial reduction in the integral dose of healthy tissues with decreased treatment-related toxicities. An in silico study comparing IMPT and IMRT could demonstrate that IMPT can significantly reduce OAR dose in the setting of Re-RT of recurrent HNC [67].
Four studies have been published with the exclusive use of PT for recurrent HNC (Table 17.3). In a study by Romesser et al. [68], 92 patients received a median dose of 60.6 Gy relative biological effectiveness (RBE) via PSPT. One-year oncological outcomes were reported with OS of 65% and LRC of 75%. The majority of locoregional recurrences (77%) were in-field. Severe late toxicity (grade 3 or higher) was mostly related to the skin (9%) and to dysphagia (7%), and there were two patients (3%) with treatment-related death due to bleeding. Phan et al. conducted a study on 60 patients receiving IMPT, in 75% of the cases with a median dose of 66 Gy RBE [24]. The 2-year OS and LRC were 70% and 73%, respectively. The 2-year actuarial rate of severe grade 3 or higher late toxicity was 26% and was associated with a Re-RT treatment volume of >50 cm3. Two patients (3%) receiving PT Re-RT to the pharynx (3%) died of potentially treatment-related toxicities. In another series of 61 patients by McDonald et al., PSPT was used to deliver a median of 66 Gy RBE for patients with a microscopic and 70.2 Gy RBE for patients with a gross disease. The median reported OS was 16.5 months with a 2-year OS of 32.7% and LRC of 80.3%. Local failure was associated with larger tumor volumes and lower Re-RT doses (continuous).
Overall, Re-RT taking advantage of PT with a reported 2-year LRC in the range from 50% to 80% and severe late toxicities between 20% and 25% seems promising. Further studies are warranted to get a better understanding of this treatment modality and to compare the results with IMRT trials.
Brachytherapy
Brachytherapy (BT) is a kind of internal radiotherapy that involves the placement of short-range radiation sources inside body cavities or interstitially. BT provides advantages over external beam radiotherapy by focussing high radiation doses to tumor volumes while minimizing the radiation exposure of healthy tissue by its steep dose gradients. BT can be differentiated by the dose rate of the used radiation source: (1) Low-dose rate (LDR) with a dose rate of up to 2 Gy/h, (2) Medium-dose rate with 2–12 Gy/h, and (3) High-dose-rate with >12 Gy/h. Possible radiation sources are the radioisotopes iodine-125 (125I) or iridium-192 (192Ir).
BT can be used for isolated nodal relapses in the neck, which occur in 10% of patients following curative treatment of HNC [71,72,73]. In a systematic review by Tselis et al., 686 patients from 12 retrospective studies have been analyzed who received BT with a median dose in the range of 30–70 Gy (Table 17.4) [74]. All studies except one used 192Ir as a radiation source. In this patient population, a 2-year OS of 13%–57% and LC of 26%–67% could be achieved while the observed grade 3 or higher late toxicity was in the range of 4–14%. The authors concluded that CT-guided HDR-BT is a treatment modality that can play an important role in the management of inoperable recurrent neck disease providing palliation and acceptable tumor control. However, the caveat of this review is the low number of patients per study (range 17–164) and the possible biases arising from the retrospective evaluation in terms of patient heterogeneity and unbalanced competing risk factors.
Take Home Message for Radiation Techniques
-
All modern radiation techniques allow an effective sparing of healthy tissue which is an important strategy to reduce significant toxicities observed with Re-RT.
-
In selected patients, durable tumor control can be achieved.
-
SBRT should be used with caution if recurrences are located within critical healthy tissues.
-
PT compared with photons has potential advantages for toxicity reduction which should be evaluated in RCTs.
-
Brachytherapy can play an important role in local control and palliation of recurrent disease of small volume irrespective of the site.
Toxicities of re-Irradiation
Acute and in particular late toxicities experienced by patients after Re-RT have a significant impact on the QoL and can even endanger their lives. Data on tolerance doses for Re-RT are scarce. However, prognostic factors predicting toxicities are known and have been previously discussed in this chapter (18.1). Dionisi et al. conducted a pooled analysis of 39 studies comprising 3766 patients on the topic of treatment-related side effects and organ tolerances after Re-RT [75]. Studies included were mostly retrospective (n = 31, 79.5%) but also of randomized (n = 3, 7.7%) and prospective (n = 5, 12.8%) designs. Regarding the treatment modality, the analysis comprised heterogeneous treatment modalities as CRT (n = 10, 25.6%), IMRT (n = 26, 66.6%), SBRT (n = 5, 12.8%), and brachytherapy (n = 2, 5.1%). Data on acute toxicities could be analyzed from 38 studies. Grade 3 or higher acute toxicities were observed in 32% of the patients (n = 1193) with 0.9% treatment-related deaths (n = 37) due to neutropenia, fatal hemorrhages, and aspiration pneumonia. No difference in the rate of acute toxicities grade 3 or higher could be observed depending on radiation techniques, Re-RT, and cumulative dose or fractionation. Severe grade 3 or higher late toxicities were observed in 29.3% (95 CI [23.5–36.4%]) of the patients (Fig. 17.4). In the pooled analysis, the risk for treatment-related death was generally low (<5%), but some series reported rates >20%. A common cause of Re-RT-related deaths was fatal hemorrhage caused by a carotid blowout.
Pooled analysis of 35 Re-RT studies on severe grade 3 or higher toxicities revealing an average rate of 29.3% and showing a general picture of expected toxicities after re-treatment [75]. Abbreviations: ES effect size
A model for prediction of grade 3 or higher toxicities after Re-RT has been developed by Ward et al. based on a retrospective study from nine institutions [50]. Patients (n = 505) received Re-RT with IMRT with a median dose of 66 Gy and outcomes were analyzed to generate a multivariable competing-risk model. A nomogram for a 2-year severe late toxicity prediction has been created, which can be integrated into the informed decision-making process of individual patients (Fig. 17.5). An additional aim was to assess whether the risk of late toxicities outweighs the risk of progression or death. Severe late toxicity with grade ≥ 3 had a 2-year incidence of 16.7% (95% CI 13.2–20.2%), while the risk for tumor progression or death was 64.2% (95% CI 59.7–68.8%). The risk of tumor progression or death is approximately four times higher than the risk of developing grade 3 or higher late toxicities after Re-RT, so ultimately, patients have to prioritize their needs based on this information.
Nomogram for prediction of grade 3 or higher late toxicities at 2 years after completion of Re-RT with IMRT [50]
Take Home Message for Toxicities of re-Irradiation
-
There is a significant risk in the range of approximately 30% for developing severe grade 3 late radiation toxicities from Re-RT including treatment related death.
-
However, the majority of patients will experience tumor progression or death before encountering severe sequels.
-
Nomograms for prediction of severe late toxicity can be integrated into the patient education.
References
Pignon J-P, Le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14.
Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368(9538):843–54.
Lacas B, Bourhis J, Overgaard J, Zhang Q, Grégoire V, Nankivell M, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18(9):1221–37.
Bourhis J, Le M, Baujat B, Audry H, Pignon J, Meta-Analysis of Chemotherapy in Head NCCG, Meta-Analysis of Radiotherapy in Carcinoma of Head NCG, Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma Collaborative Group. Individual patients’ data meta-analyses in head and neck cancer. Curr Opin Oncol. 2007;19(3):188–94.
Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004 Aug 1;15(8):1179–86.
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with Docetaxel in head and neck cancer. N Engl J Med. 2007 Oct 25;357(17):1705–15.
Farrag A, Voordeckers M, Tournel K, De Coninck P, Storme G. Pattern of failure after helical tomotherapy in head and neck cancer. Strahlenther Onkol. 2010 Sep;186(9):511–6.
Dawson LA, Anzai Y, Marsh L, Martel MK, Paulino A, Ship JA, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2000;46(5):1117–26.
Chao KSC, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003 Feb 1;55(2):312–21.
Salama JK, Vokes EE, Chmura SJ, Milano MT, Kao J, Stenson KM, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006 Feb 1;64(2):382–91.
Langer CJ, Harris J, Horwitz EM, Nicolaou N, Kies M, Curran W, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of radiation therapy oncology group protocol 9911. J Clin Oncol. 2007 Oct 20;25(30):4800–5.
Spencer SA, Harris J, Wheeler RH, Machtay M, Schultz C, Spanos W, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008 Mar;30(3):281–8.
Strojan P, Corry J, Eisbruch A, Vermorken JB, Mendenhall WM, Lee AWM, et al. Recurrent and second primary squamous cell carcinoma of the head and neck: when and how to reirradiate. Head Neck. 2015;37(1):134–50.
Burnet NG, Johansen J, Turesson I, Nyman J, Peacock JH. Describing patients’ normal tissue reactions: concerning the possibility of individualising radiotherapy dose prescriptions based on potential predictive assays of normal tissue radiosensitivity. Int J Cancer. 1998;79(6):606–13.
Tanvetyanon T, Padhya T, McCaffrey J, Zhu W, Boulware D, DeConti R, et al. Prognostic factors for survival after salvage Reirradiation of head and neck cancer. JCO. 2009 Mar 16;27(12):1983–91.
Kodani N, Yamazaki H, Tsubokura T, Shiomi H, Kobayashi K, Nishimura T, et al. Stereotactic body radiation therapy for head and neck tumor: disease control and morbidity outcomes. J Radiat Res. 2011;52(1):24–31.
Rwigema J-CM, Heron DE, Ferris RL, Andrade RS, Gibson MK, Yang Y, et al. The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol. 2011 Aug;34(4):372–9.
Vargo JA, Ferris RL, Ohr J, Clump DA, Davis KS, Duvvuri U, et al. A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2015;91(3):480–8.
Vargo JA, Heron DE, Ferris RL, Rwigema JM, Kalash R, Wegner RE, et al. Examining tumor control and toxicity after stereotactic body radiotherapy in locally recurrent previously irradiated head and neck cancers: implications of treatment duration and tumor volume. Head Neck. 2014;36(9):1349–55.
Chen AM, Farwell DG, Luu Q, Cheng S, Donald PJ, Purdy JA. Prospective trial of high-dose reirradiation using daily image guidance with intensity-modulated radiotherapy for recurrent and second primary head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80(3):669–76.
Yamazaki H, Ogita M, Himei K, Nakamura S, Suzuki G, Yoshida K, et al. Reirradiation using robotic image-guided stereotactic radiotherapy of recurrent head and neck cancer. J Radiat Res. 2016;57(3):288–93.
Leung T-W, Tung SY, Sze W-K, Sze W-M, Wong VYW, Wong C-S, et al. Salvage radiation therapy for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000 Dec 1;48(5):1331–8.
Takiar V, Garden AS, Ma D, Morrison WH, Edson M, Zafereo ME, et al. Reirradiation of head and neck cancers with intensity modulated radiation therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys. 2016 Jul 15;95(4):1117–31.
Phan J, Sio TT, Nguyen TP, Takiar V, Gunn GB, Garden AS, et al. Reirradiation of head and neck cancers with proton therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys. 2016 Sep 1;96(1):30–41.
Chen AM, Phillips TL, Lee NY. Practical considerations in the re-irradiation of recurrent and second primary head-and-neck cancer: who, why, how, and how much? Int J Radiat Oncol Biol Phys. 2011 Dec 1;81(5):1211–9.
Duprez F, Madani I, Bonte K, Boterberg T, Vakaet L, Derie C, et al. Intensity-modulated radiotherapy for recurrent and second primary head and neck cancer in previously irradiated territory. Radiother Oncol. 2009 Dec 1;93(3):563–9.
Chua DTT, Sham JST, Hung K-N, Leung LHT, Au GKH. Predictive factors of tumor control and survival after radiosurgery for local failures of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006 Dec 1;66(5):1415–21.
Lee N, Chan K, Bekelman JE, Zhung J, Mechalakos J, Narayana A, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007 Jul 1;68(3):731–40.
Dawson LA, Myers LL, Bradford CR, Chepeha DB, Hogikyan ND, Teknos TN, et al. Conformal re-irradiation of recurrent and new primary head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001 Jun 1;50(2):377–85.
Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma--treatment techniques and results. Int J Radiat Oncol Biol Phys. 1987 Jul;13(7):953–6.
Wang CC, McIntyre J. Re-irradiation of laryngeal carcinoma—techniques and results. Int J Radiat Oncol Biol Phys. 1993 Aug 1;26(5):783–5.
Orlandi E, Bonomo P, Ferella L, D’Angelo E, Maddalo M, Alterio D, et al. Long-term outcome of re-irradiation for recurrent or second primary head and neck cancer: a multi-institutional study of AIRO-head and neck working group. Head Neck. 2019;41(10):3684–92.
Law SCK, Lam W-K, Ng M-F, Au S-K, Mak W-T, Lau W-H. Reirradiation of nasopharyngeal carcinoma with intracavitary mold brachytherapy: an effective means of local salvage. Int J Radiat Oncol Biol Phys. 2002 Nov 15;54(4):1095–113.
Spencer SA, Harris J, Wheeler RH, Machtay M, Schultz C, Spanos W, et al. RTOG 96-10: reirradiation with concurrent hydroxyurea and 5-fluorouracil in patients with squamous cell cancer of the head and neck. Int J Radiat Oncol Biol Phys. 2001 Dec 1;51(5):1299–304.
Stevens KR, Britsch A, Moss WT. High-dose reirradiation of head and neck cancer with curative intent. Int J Radiat Oncol Biol Phys. 1994 Jul 1;29(4):687–98.
Janot F, de Raucourt D, Benhamou E, Ferron C, Dolivet G, Bensadoun R-J, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008 Dec 1;26(34):5518–23.
Sulman EP, Schwartz DL, Le TT, Ang KK, Morrison WH, Rosenthal DI, et al. IMRT Reirradiation of head and neck cancer—disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys. 2009 Feb 1;73(2):399–409.
Spencer SA, Wheeler RH, Peters GE, Beenken SW, Meredith RF, Smith J, et al. Concomitant chemotherapy and reirradiation as management for recurrent cancer of the head and neck. Am J Clin Oncol. 1999 Feb;22(1):1–5.
Langendijk JA, Bourhis J. Reirradiation in squamous cell head and neck cancer: recent developments and future directions. Curr Opin Oncol. 2007 May;19(3):202–9.
Rwigema J-C, Heron DE, Ferris RL, Gibson M, Quinn A, Yang Y, et al. Fractionated stereotactic body radiation therapy in the treatment of previously-irradiated recurrent head and neck carcinoma: updated report of the University of Pittsburgh experience. Am J Clin Oncol. 2010 Jun;33(3):286–93.
Caudell JJ, Ward MC, Riaz N, Zakem SJ, Awan MJ, Dunlap NE, et al. Volume, dose, and fractionation considerations for IMRT-based Reirradiation in head and neck cancer: a multi-institution analysis. Int J Radiat Oncol Biol Phys. 2018;100(3):606–17.
Popovtzer A, Gluck I, Chepeha DB, Teknos TN, Moyer JS, Prince ME, et al. The pattern of failure after re-irradiation of recurrent squamous cell head and neck cancer: implications for defining the targets. Int J Radiat Oncol Biol Phys. 2009 Aug 1;74(5):1342–7.
Sher DJ, Haddad RI, Norris CM, Posner MR, Wirth LJ, Goguen LA, et al. Efficacy and toxicity of reirradiation using intensity-modulated radiotherapy for recurrent or second primary head and neck cancer. Cancer. 2010 Oct 15;116(20):4761–8.
Biagioli MC, Harvey M, Roman E, Raez LE, Wolfson AH, Mutyala S, et al. Intensity-modulated radiotherapy with concurrent chemotherapy for previously irradiated, recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007 Nov 15;69(4):1067–73.
Kharofa J, Choong N, Wang D, Firat S, Schultz C, Sadasiwan C, et al. Continuous-course reirradiation with concurrent carboplatin and paclitaxel for locally recurrent, nonmetastatic squamous cell carcinoma of the head-and-neck. Int J Radiat Oncol Biol Phys. 2012 Jun 1;83(2):690–5.
Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010 Oct 1;21:vii252–61.
Zwicker F, Roeder F, Hauswald H, Thieke C, Timke C, Schlegel W, et al. Reirradiation with intensity-modulated radiotherapy in recurrent head and neck cancer. Head Neck. 2011 Dec;33(12):1695–702.
Duprez F, Berwouts D, Madani I, Bonte K, Boterberg T, De Gersem W, et al. High-dose reirradiation with intensity-modulated radiotherapy for recurrent head-and-neck cancer: disease control, survival and toxicity. Radiother Oncol. 2014 Jun;111(3):388–92.
Curtis KK, Ross HJ, Garrett AL, Jizba TA, Patel AB, Patel SH, et al. Outcomes of patients with loco-regionally recurrent or new primary squamous cell carcinomas of the head and neck treated with curative intent reirradiation at Mayo Clinic. Radiat Oncol. 2016 Apr 9;11:55.
Ward MC, Lee NY, Caudell JJ, Zajichek A, Awan MJ, Koyfman SA, et al. A competing risk nomogram to predict severe late toxicity after modern re-irradiation for squamous carcinoma of the head and neck. Oral Oncol. 2019 Mar 1;90:80–6.
Tao Y, Faivre L, Laprie A, Boisselier P, Ferron C, Jung GM, et al. Randomized trial comparing two methods of re-irradiation after salvage surgery in head and neck squamous cell carcinoma: once daily split-course radiotherapy with concomitant chemotherapy or twice daily radiotherapy with cetuximab. Radiother Oncol. 2018 Sep 1;128(3):467–71.
Comet B, Kramar A, Faivre-Pierret M, Dewas S, Coche-Dequeant B, Degardin M, et al. Salvage stereotactic reirradiation with or without cetuximab for locally recurrent head-and-neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2012 Sep 1;84(1):203–9.
Marta GN, Silva V, de Andrade Carvalho H, de Arruda FF, Hanna SA, Gadia R, et al. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol. 2014;110(1):9–15.
Chen Y-J, Kuo JV, Ramsinghani NS, Al-Ghazi MSAL. Intensity-modulated radiotherapy for previously irradiated, recurrent head-and-neck cancer. Med Dosim. 2002;27(2):171–6.
Vargo JA, Ward MC, Caudell JJ, Riaz N, Dunlap NE, Isrow D, et al. A multi-institutional comparison of SBRT and IMRT for definitive Reirradiation of recurrent or second primary head and neck cancer. Int J Radiat Oncol Biol Phys. 2018 Mar 1;100(3):595–605.
Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A radiation therapy oncology group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000 Aug 1;48(1):7–16.
Lartigau EF, Tresch E, Thariat J, Graff P, Coche-Dequeant B, Benezery K, et al. Multi institutional phase II study of concomitant stereotactic reirradiation and cetuximab for recurrent head and neck cancer. Radiother Oncol. 2013 Nov;109(2):281–5.
Román AA, Jodar C, Perez-Rozos A, Lupiañez-Perez Y, Medina JA, Gomez-Millan J. The role of stereotactic body radiotherapy in reirradiation of head and neck cancer recurrence. Crit Rev Oncol Hematol. 2018 Feb 1;122:194–201.
Yazici G, Sanlı TY, Cengiz M, Yuce D, Gultekin M, Hurmuz P, et al. A simple strategy to decrease fatal carotid blowout syndrome after stereotactic body reirradiaton for recurrent head and neck cancers. Radiat Oncol. 2013 Oct 18;8:242.
Siddiqui F, Patel M, Khan M, McLean S, Dragovic J, Jin J-Y, et al. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2009 Jul 15;74(4):1047–53.
Roh K-W, Jang J-S, Kim M-S, Sun D-I, Kim B-S, Jung S-L, et al. Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2009;74(5):1348–55.
Heron DE, Ferris RL, Karamouzis M, Andrade RS, Deeb EL, Burton S, et al. Stereotactic body radiotherapy for recurrent squamous cell carcinoma of the head and neck: results of a phase I dose-escalation trial. Int J Radiat Oncol Biol Phys. 2009 Dec 1;75(5):1493–500.
Unger KR, Lominska CE, Deeken JF, Davidson BJ, Newkirk KA, Gagnon GJ, et al. Fractionated stereotactic radiosurgery for reirradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010 Aug 1;77(5):1411–9.
Cengiz M, Özyiğit G, Yazici G, Doğan A, Yildiz F, Zorlu F, et al. Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2011 Sep 1;81(1):104–9.
Iwata H, Tatewaki K, Inoue M, Yokota N, Sato K, Shibamoto Y. Salvage stereotactic reirradiation using the CyberKnife for the local recurrence of nasal or paranasal carcinoma. Radiother Oncol. 2012 Sep;104(3):355–60.
Kress M-AS, Sen N, Unger KR, Lominska CE, Deeken JF, Davidson BJ, et al. Safety and efficacy of hypofractionated stereotactic body reirradiation in head and neck cancer: long-term follow-up of a large series. Head Neck. 2015;37(10):1403–9.
Eekers DBP, Roelofs E, Jelen U, Kirk M, Granzier M, Ammazzalorso F, et al. Benefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trial. Radiother Oncol. 2016 Dec 1;121(3):387–94.
Romesser PB, Cahlon O, Scher ED, Hug EB, Sine K, DeSelm C, et al. Proton beam re-irradiation for recurrent head and neck cancer: multi-institutional report on feasibility and early outcomes. Int J Radiat Oncol Biol Phys. 2016 May 1;95(1):386–95.
Lin R, Slater JD, Yonemoto LT, Grove RI, Teichman SL, Watt DK, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy--dose-volume histogram analysis. Radiology. 1999 Nov;213(2):489–94.
McDonald MW, Zolali-Meybodi O, Lehnert SJ, Estabrook NC, Liu Y, Cohen-Gadol AA, et al. Reirradiation of recurrent and second primary head and neck cancer with proton therapy. Int J Radiat Oncol Biol Phys. 2016 15;96(4):808–19.
Choo R, Grimard L, Esche B, Crook J, Genest P, Odell P. Brachytherapy of neck metastases. J Otolaryngol. 1993;22(1):54–7.
Fazekas JT, Sommer C, Kramer S. Tumor regression and other prognosticators in advanced head and neck cancers: a sequel to the RTOG methotrexate study. Int J Radiat Oncol Biol Phys. 1983 Jul 1;9(7):957–64.
Gerbaulet A, Michel G, Haie-Meder C, Castaigne D, Lartigau E, L’Homme C, et al. The role of low dose rate brachytherapy in the treatment of cervix carcinoma. Experience of the Gustave-Roussy institute on 1245 patients. Eur J Gynaecol Oncol. 1995;16(6):461–75.
Tselis N, Ratka M, Vogt H-G, Kolotas C, Baghi M, Baltas D, et al. Hypofractionated accelerated CT-guided interstitial 192Ir-HDR-brachytherapy as re-irradiation in inoperable recurrent cervical lymphadenopathy from head and neck cancer. Radiother Oncol. 2011 Jan 1;98(1):57–62.
Dionisi F, Fiorica F, D’Angelo E, Maddalo M, Giacomelli I, Tornari E, et al. Organs at risk’s tolerance and dose limits for head and neck cancer re-irradiation: a literature review. Oral Oncol. 2019 Nov 1;98:35–47.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this paper
Cite this paper
Budach, V., Thieme, A. (2021). Re-Irradiation for Local Relapses or Second Primaries: When and how?. In: Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, JP., Nicolai, P., O’Sullivan, B. (eds) Critical Issues in Head and Neck Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-63234-2_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-63234-2_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63233-5
Online ISBN: 978-3-030-63234-2
eBook Packages: MedicineMedicine (R0)