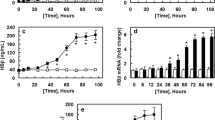
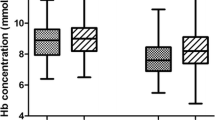
Abstract
Sepsis is a heterogeneous clinical syndrome that has defied all attempts to identify effective pharmacologic therapies. A growing body of evidence suggests that an elevated level of circulating cell-free hemoglobin is a feature of some, but not all patients with sepsis. Cell-free hemoglobin can cause tissue injury and organ dysfunction through a variety of injurious mechanisms including scavenging of nitric oxide, pro-inflammatory signaling, and oxidative injury. The kidney and lung appear to be particularly vulnerable to cell-free hemoglobin-mediated injury. A number of potential therapies to target cell-free hemoglobin in sepsis have been identified including haptoglobin, hemopexin, and acetaminophen. Early phase clinical trials suggest that acetaminophen may have beneficial effects on lipid peroxidation and kidney function in patients with sepsis. Measurement of cell-free hemoglobin levels at the bedside has the potential to facilitate predictive enrichment for future therapeutic trials of cell-free hemoglobin-targeted therapeutics in sepsis such that only patients with elevated cell-free hemoglobin who would be most likely to benefit would be enrolled. However, rapid, accurate bedside tests for plasma cell-free hemoglobin will need to be developed in order for such trials to move forward.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54.
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–10.
Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194:147–55.
US Food and Drug Administration. Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products: Guidance for Industry. Available at https://www.fda.gov/media/121320/download. Accessed 7 Sept 2019.
Janz DR, Bastarache JA, Peterson JF, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41:784–90.
Adamzik M, Hamburger T, Petrat F, et al. Free hemoglobin concentration in severe sepsis: methods of measurement and prediction of outcome. Crit Care. 2012;16:R125.
Cooper GS, Havlir DS, Shlaes DM, Salata RA. Polymicrobial bacteremia in the late 1980s: predictors of outcome and review of the literature. Medicine (Baltimore). 1990;69:114–23.
Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:51ra71.
Effenberger-Neidnicht K, Hartmann M. Mechanisms of hemolysis during sepsis. Inflammation. 2018;41:1569–81.
Janz DR, Bastarache JA, Sills G, et al. Association between haptoglobin, hemopexin and mortality in adults with sepsis. Crit Care. 2013;17:R272.
Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–84.
Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci U S A. 2010;107:2699–704.
Shaver CM, Wickersham N, McNeil JB, et al. Cell-free hemoglobin promotes primary graft dysfunction through oxidative lung endothelial injury. JCI Insight. 2018;3:e98546.
Shaver CM, Upchurch CP, Janz DR, et al. Cell-free hemoglobin: a novel mediator of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;310:L532–41.
Mumby S, Ramakrishnan L, Evans TW, Griffiths MJ, Quinlan GJ. Methemoglobin-induced signaling and chemokine responses in human alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2014;306:L88–100.
Chintagari NR, Jana S, Alayash AI. Oxidized ferric and ferryl forms of hemoglobin trigger mitochondrial dysfunction and injury in alveolar type I cells. Am J Respir Cell Mol Biol. 2016;55:288–98.
Plewes K, Kingston HWF, Ghose A, et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis. 2017;17:313.
Shaver CM, Paul MG, Putz ND, et al. Cell-free hemoglobin augments acute kidney injury during experimental sepsis. Am J Physiol Renal Physiol. 2019;317:F922–9.
Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol. 2015;35:2–11.
Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20:588–95.
Baek JH, D'Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–58.
Baek JH, Zhang X, Williams MC, et al. Extracellular Hb enhances cardiac toxicity in endotoxemic guinea pigs: protective role of haptoglobin. Toxins (Basel). 2014;6:1244–59.
Graw JA, Mayeur C, Rosales I, et al. Haptoglobin or hemopexin therapy prevents acute adverse effects of resuscitation after prolonged storage of red cells. Circulation. 2016;134:945–60.
Remy KE, Cortes-Puch I, Solomon SB, et al. Haptoglobin improves shock, lung injury, and survival in canine pneumonia. JCI Insight. 2018;3:e123013.
MohanKumar K, Namachivayam K, Song T, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun. 2019;10:3494.
Tanaka K, Kanamori Y, Sato T, et al. Administration of haptoglobin during cardiopulmonary bypass surgery. ASAIO Trans. 1991;37:M482–3.
Nomura K, Hashimoto K, Miyamoto N, et al. Hemolytic renal damage during cardiopulmonary bypass and the preventive effect of haptoglobin. Jpn J Cadiovasc Surg. 1993;22:404–8.
Kubota K, Egi M, Mizobuchi S. Haptoglobin administration in cardiovascular surgery patients: its association with the risk of postoperative acute kidney injury. Anesth Analg. 2017;124:1771–6.
Miller YI, Smith A, Morgan WT, Shaklai N. Role of hemopexin in protection of low-density lipoprotein against hemoglobin-induced oxidation. Biochemistry (Mosc). 1996;35:13112–7.
Jung JY, Kwak YH, Kim KS, Kwon WY, Suh GJ. Change of hemopexin level is associated with the severity of sepsis in endotoxemic rat model and the outcome of septic patients. J Crit Care. 2015;30:525–30.
Elphinstone RE, Conroy AL, Hawkes M, et al. Alterations in systemic extracellular heme and hemopexin are associated with adverse clinical outcomes in Ugandan children with severe malaria. J Infect Dis. 2016;214:1268–75.
Lin T, Maita D, Thundivalappil SR, et al. Hemopexin in severe inflammation and infection: mouse models and human diseases. Crit Care. 2015;19:166.
Jung JY, Kwak YH, Chang I, et al. Protective effect of hemopexin on systemic inflammation and acute lung injury in an endotoxemia model. J Surg Res. 2017;212:15–21.
Ouellet M, Percival MD. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch Biochem Biophys. 2001;387:273–80.
Anderson BJ. Paracetamol (Acetaminophen): mechanisms of action. Paediatr Anaesth. 2008;18:915–21.
Gonzalez-Sanchez MI, Manjabacas MC, Garcia-Carmona F, Valero E. Mechanism of acetaminophen oxidation by the peroxidase-like activity of methemoglobin. Chem Res Toxicol. 2009;22:1841–50.
Boutaud O, Roberts LJ 2nd. Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic Biol Med. 2011;51:1062–7.
Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012:CD006616.
Roberts LJ 2nd, Oates JA, Linton MF, et al. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med. 2007;43:1388–93.
Blumberg JB, Frei B. Why clinical trials of vitamin E and cardiovascular diseases may be fatally flawed. Commentary on “The relationship between dose of vitamin E and suppression of oxidative stress in humans”. Free Radic Biol Med. 2007;43:1374–6.
Van Driest SL, Jooste EH, Shi Y, et al. Association between early postoperative acetaminophen exposure and acute kidney injury in pediatric patients undergoing cardiac surgery. JAMA Pediatr. 2018;172:655–63.
Janz DR, Bastarache JA, Rice TW, et al. Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: the Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis trial. Crit Care Med. 2015;43:534–41.
Simpson SA, Zaccagni H, Bichell DP, et al. Acetaminophen attenuates lipid peroxidation in children undergoing cardiopulmonary bypass. Pediatr Crit Care Med. 2014;15:503–10.
Billings FT, Petracek MR, Roberts LJ 2nd, Pretorius M. Perioperative intravenous acetaminophen attenuates lipid peroxidation in adults undergoing cardiopulmonary bypass: a randomized clinical trial. PLoS One. 2015;10:e0117625.
Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe Falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67:991–9.
Kuck JL, Bastarache JA, Shaver CM, et al. Ascorbic acid attenuates endothelial permeability triggered by cell-free hemoglobin. Biochem Biophys Res Commun. 2018;495:433–7.
Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin c, and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest. 2017;151:1229–38.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ware, L.B. (2020). Cell-Free Hemoglobin: A New Therapeutic Target in Sepsis?. In: Vincent, JL. (eds) Annual Update in Intensive Care and Emergency Medicine 2020. Annual Update in Intensive Care and Emergency Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-37323-8_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-37323-8_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-37322-1
Online ISBN: 978-3-030-37323-8
eBook Packages: MedicineMedicine (R0)