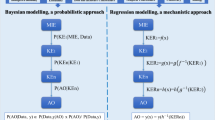
Abstract
The correlation of chemical structure with physicochemical and biological data to assess a desired or undesired biological outcome now utilises both qualitative and quantitative structure–activity relationships ((Q)SARs) and advanced computational methods . The adoption of in silico methodologies for predicting toxicity, as decision support tools, is now a common practice in both developmental and regulatory contexts for certain toxicity endpoints. The relative success of these tools has unveiled further challenges relating to interpreting and applying the results of models. These include the concept of what makes a negative prediction and exploring the use of test data to make quantitative predictions. Due to several factors, including the lack of understanding of mechanistic pathways in biological systems, modelling complex endpoints such as organ toxicity brings new challenges. The use of the adverse outcome pathway (AOP) framework as a construct to arrange models and data, to tackle such challenges, is reviewed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- (Q)SAR:
-
(Quantitative) structure–activity relationship
- ADME:
-
Adsorption, distribution, metabolism, and excretion
- AOP:
-
Adverse outcome pathway
- BSEP:
-
Bile salt export pump
- DA:
-
Defined approach
- DMSO:
-
Dimethyl sulphoxide
- DNA:
-
Deoxyribonucleic acid
- DPRA:
-
Direct peptide reactivity assay
- EC3:
-
Effective concentration to cause a threefold increase in T-cell proliferation
- GHS:
-
Globally harmonised system
- GPMT:
-
Guinea pig maximisation test
- h-CLAT:
-
Human cell line activation test
- IATA:
-
Integrated approach to testing and assessment
- KE:
-
Key event
- kNN:
-
k-Nearest neighbours
- LLNA:
-
Local lymph node assay
- MIE:
-
Molecular initiating event
- MW:
-
Molecular weight
- OATP:
-
Organic anion transporting polypeptide
- OECD:
-
Organisation for Economic Co-operation and Development
- PPAR:
-
Peroxisome proliferator-activated receptor
References
Ashby J, Tennant RW (1988) Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat Res 204:17–115
Marchant C et al (2008) In silico tools for sharing data and knowledge on toxicity and metabolism: derek for windows, meteor, and vitic. Toxicol Mech Methods 18:177–187
Barber C et al (2015) Establishing best practise in the application of expert review of mutagenicity under ICH M7. Regul Toxicol Pharmacol 73:367–377
Hanser T et al (2014) Self organising hypothesis networks: a new approach for representing and structuring SAR knowledge. J Chemoinf 6:21
Barber C et al (2017) Distinguishing between expert and statistical systems for application under ICH M7. Regul Toxicol Pharmacol 84:124–130
Ankley GT et al (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741
OECD (2010) Test No. 429: skin sensitisation: local lymph node assay. https://doi.org/10.1787/9789264071100-en. Accessed 28 Aug 2018
ICH (2017) Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. M7(R1). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M7/M7_R1_Addendum_Step_4_31Mar2017.pdf. Accessed 9 Sept 2018
EFSA (2016) Guidance on the establishment of the residue definition for dietary risk assessment. EFSA J 14: 180. https://doi.org/10.2903/j.efsa.2016.4549. Accessed 9 Sept 2018
OECD (2017) Guidance document on the reporting of defined approaches and individual information sources to be used within Integrated Approaches to Testing and Assessment (IATA) for skin sensitisation. In: OECD series on testing and assessment, No. 256. OECD Publishing, Paris. https://doi.org/10.1787/9789264279285-en. Accessed 28 Aug 2018
European Union (2009) Regulation (EC) No 1223/2009 of the European Parliament and of the council of 30 November 2009 on cosmetic products. http://data.europa.eu/eli/reg/2009/1223/oj. Accessed 28 Aug 2018
European Union (2006) Regulation (EC) No 1907/2006 of the European Parliament and of the council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. http://data.europa.eu/eli/reg/2006/1907/2018-05-09. Accessed 28 Aug 2018
Elder DP et al (2015) Mutagenic impurities: precompetitive/competitive collaborative and data sharing initiatives. Org Process Res Dev 19:1486–1494
Judson PN et al (2013) Assessing confidence in predictions made by knowledge-based systems. Toxicol Res 2:70–79
Williams RV et al (2016) It’s difficult, but important, to make negative predictions. Regul Toxicol Pharmacol 76:79–86
Derek Nexus v6.0 (Lhasa Limited). https://www.lhasalimited.org/products/derek-nexus.htm. Accessed 28 Aug 2018
Chilton ML et al (2018) Making reliable negative predictions of human skin sensitisation using an in silico fragmentation approach. Regul Toxicol Pharmacol 95:227–235
Canipa SJ et al (2016) A quantitative in silico model for predicting skin sensitization using a nearest neighbours approach within expert-derived structure-activity alert spaces. J Appl Toxicol 37:985–995
OECD (2015) Test No. 442C: In chemico skin sensitisation: Direct Peptide Reactivity Assay (DPRA). https://doi.org/10.1787/9789264229709-en. Accessed 28 Aug 2018
OECD (2018) Key event based test guideline 442D: in vitro skin sensitisation assays addressing the AOP key event on keratinocyte activation. https://doi.org/10.1787/9789264229822-en. Accessed 28 Aug 2018
OECD (2018) Key event based test guideline 442E: In vitro skin sensitisation assays addressing the key event on activation of dendritic cells on the adverse outcome pathway for skin sensitisation. https://doi.org/10.1787/9789264264359-en. Accessed 28 Aug 2018
Kleinstreuer NC et al (2018) Non-animal methods to predict skin sensitization (II): an assessment of defined approaches. Crit Rev Toxicol 48:359–374
Macmillan DS et al (2016) Predicting skin sensitisation using a decision tree integrated testing strategy with an in silico model and in chemico/in vitro assays. Regul Toxicol Pharmacol 76:30–38
Barber C et al (2016) Establishing best practise in the application of expert review of mutagenicity under ICH M7. Regul Toxicol Pharmacol 73:367–377
Verheyen GR et al (2017) Evaluation of in silico tools to predict the skin sensitization potential of chemicals. SAR QSAR Environ Res 28:59–73
Dobo KL et al (2012) In silico methods combined with expert knowledge rule out mutagenic potential of pharmaceutical impurities: an industry survey. Regul Toxicol Pharmacol 62:449–455
Kruhlak NL et al (2012) (Q)SAR modeling and safety assessment in regulatory review. Clin Pharmacol Ther 91:529–534
Naven RT et al (2012) Latest advances in computational genotoxicity prediction. Expert Opin Drug Metab Toxicol 8:1579–1587
Sutter A et al (2013) Use of in silico systems and expert knowledge for structure-based assessment of potentially mutagenic impurities. Regul Toxicol Pharmacol 67:39–52
Powley MW (2015) (Q)SAR assessments of potentially mutagenic impurities: a regulatory perspective on the utility of expert knowledge and data submission. Regul Toxicol Pharmacol 71:295–300
Greene N et al (2015) A practical application of two in silico systems for identification of potentially mutagenic impurities. Regul Toxicol Pharmacol 72:335–349
Amberg A et al (2016) Principles and procedures for implementation of ICH M7 recommended (Q)SAR analyses. Regul Toxicol Pharmacol 77:13–24
Myatt GJ et al (2018) In silico toxicology protocols. Regul Toxicol Pharmacol 96:1–17
Roberts DW et al (2016) Chemical applicability domain of the Local Lymph Node Assay (LLNA) for skin sensitisation potency. Part 3. Apparent discrepancies between LLNA and GPMT sensitisation potential: false positives or differences in sensitivity? Regul Toxicol Pharmacol 80:260–267
Honda H et al (2016) Modified Ames test using a strain expressing human sulfotransferase 1C2 to assess the mutagenicity of methyleugenol. Genes Environ. https://doi.org/10.1186/s41021-016-0028-x
Amberg A et al (2015) Do carboxylic/sulfonic acid halides really present a mutagenic and carcinogenic risk as impurities in final drug products? Org Process Res Dev 19:1495–1506
Sarah Nexus v3.0 (Lhasa Limited). https://www.lhasalimited.org/products/sarah-nexus.htm. Accessed 28 Aug 2018
Myden A et al (2017) Utility of published DNA reactivity alerts. Regul Toxicol Pharmacol 88:77–86
Faulkner D et al (2017) Tools for green molecular design to reduce toxicological risk. In: Johnson DE, Richardson RJ (eds) Computational systems pharmacology and toxicology, Royal Society of Chemistry, London, Chapter 3, p 36–59
Canipa S et al (2015) Using in vitro structural alerts for chromosome damage to predict in vivo activity and direct future testing. Mutagenesis 31:17–25
Egan WJ et al (2004) In silico prediction of drug safety: despite progress there is abundant room for improvement. Drug Discov Today Technol 1:381–387
Greene N et al (2010) Developing structure-activity relationships for the prediction of hepatotoxicity. Chem Res Toxicol 23:1215–1222
Hewitt M et al (2013) Hepatotoxicity: a scheme for generating chemical categories for read-across, structural alerts and insights into mechanism(s) of action. Crit Rev Toxicol 43:537–558
Pizzo F et al (2016) A new structure-activity relationship (SAR) model for predicting drug-induced liver injury, based on statistical and expert-based structural alerts. Front Pharmacol. https://doi.org/10.3389/fphar.2016.00442
Liu R et al (2015) Data-driven identification of structural alerts for mitigating the risk of drug-induced human liver injuries. J Cheminf. https://doi.org/10.1186/s13321-015-0053-y
Myshkin E et al (2012) Prediction of organ toxicity endpoints by QSAR modeling based on precise chemical-histopathology annotations. Chem Biol Drug Des 80:406–416
OECD (2012) AOP knowledgebase. https://aopkb.oecd.org/. Accessed 28 Aug 2018
Thompson RA et al (2016) Reactive metabolites: current and emerging risk and hazard assessments. Chem Res Toxicol 29:505–533
Warner DJ et al (2012) Mitigating the inhibition of human bile salt export pump by drugs: opportunities provided by physicochemical property modulation, in silico modeling, and structural modification. Drug Metab Dispos 40:2332–2341. https://doi.org/10.1124/dmd.112.047068
Qiu T et al (2018) Finding the molecular scaffold of nuclear receptor inhibitors through high-throughput screening based on proteochemometric modelling. J Cheminf. https://doi.org/10.1186/s13321-018-0275-x
Gadaleta D et al (2018) QSAR modeling of ToxCast assays relevant to the molecular initiating events of AOPs leading to hepatic steatosis. J Chem Inf Model 58:1501–1517
Kotsampasakou E, Ecker GF (2017) Predicting drug-induced cholestasis with the help of hepatic transporters-an in silico modeling approach. J Chem Inf Model 57:608–615
Altamira LLC, Molecular Networks GmbH (2013) ChemoTyper. https://chemotyper.org/. Accessed 28 Aug 2018
Liu J et al (2015) Predicting hepatotoxicity using ToxCast in vitro bioactivity and chemical structure. Chem Res Toxicol 28:738–751
Liu J et al (2017) Predicting organ toxicity using in vitro bioactivity data and chemical structure. Chem Res Toxicol 30:2046–2059
Carbonell P et al (2017) Hepatotoxicity prediction by systems biology modeling of disturbed metabolic pathways using gene expression data. Altex 34:219–234
Kim J, Shin M (2014) An integrative model of multi-organ drug-induced toxicity prediction using gene-expression data. BMC Bioinform. https://doi.org/10.1186/1471-2105-15-s16-s2
Alyass A et al (2015) From big data analysis to personalized medicine for all: challenges and opportunities. BMC Med Genomics. https://doi.org/10.1186/s12920-015-0108-y
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Williams, R., Chilton, M., Macmillan, D., Cayley, A., Fisk, L., Patel, M. (2019). Modelling Simple Toxicity Endpoints: Alerts, (Q)SARs and Beyond. In: Hong, H. (eds) Advances in Computational Toxicology. Challenges and Advances in Computational Chemistry and Physics, vol 30. Springer, Cham. https://doi.org/10.1007/978-3-030-16443-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-16443-0_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16442-3
Online ISBN: 978-3-030-16443-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)