Abstract
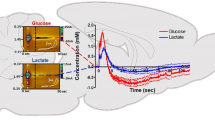
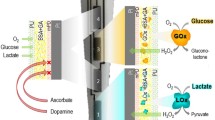
Glucose biosensors were prepared by immobilizing glucose oxidase on carbon fiber microelectrodes (CFMEs) either by cross-linking glutaraldehyde vapors or via enzyme entrapment in films of m-phenylenediamine or resorcinol. The enzymatic layer was then covered with a membrane made of Nafion or cellulose acetate. The biosensors were tested using differential normal pulse voltammetry (DNPV) to detect signals. The calibration curves for glucose were linear from 0.3 to 6.5 mM. The DNPV response was essentially insensitive to potentially interfering molecules. Glucose concentrations in plasma and cerebrospinal fluid (CSF) corresponded with those measured by standard procedures. In freely moving rats, cortical extracellular glucose concentration averaged 0.59 ± 0.3 mM. Using combined polysomnographic and DNPV assessment, glucose levels in the cortex were determined during various phases of the sleep–wake cycle. Compared to the waking state (W, 100 %), spontaneous variations were observed during active waking triggered by a water puff (AW, −32 %), slow-wave sleep (SWS, +13 %), and paradoxical sleep (PS, −11 %). To prepare lactate biosensors, CFMEs were coated with lactate oxidase. These sensors, combined with DNPV measurements, showed a linear response to lactate at concentrations ranging from 0.1 to 2.0 mM. Lactate measurement was insensitive to common potentially interfering molecules. In anesthetized rats, cortical lactate concentration averaged 0.41 ± 0.02 mM. Lactate levels in samples of brain tissue, plasma, and CSF corresponded to those measured by standard procedures. In freely moving rats, lactate changes were also related to sleep–wake state. Compared to W (100 %), lactate level increased during AW (+53 %) and decreased during SWS (−16.2 %). However, during PS, lactate level increased (+8.5 %) compared to that noted during SWS. Finally, long-term lactate monitoring revealed the existence of a circadian influence on lactate production rate.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Magistretti PJ, Pellerin L, Martin JL, Bloom FE (1995) Brain energy metabolism: an integrated cellular perspective. In: Bloom FE, Kupfer DJ (eds) Psychopharmacology: the fourth generation of progress. Raven, New York, pp 657–670
Magistretti PJ, Pellerin L, Rothman DL, Shulman RG (1999) Energy on demand. Science 283:496–497
Magistretti PJ, Allaman I (2007) Glycogen: a Trojan horse for neurons. Nat Neurosci 10:1341–1342
Cespuglio R, Colas D, Gautier-Sauvigné S (2005) Energy processes underlying the sleep-wake cycle. In: Parmeggiani PL, Velluti R (eds) The physiological nature of sleep. Imperial College Press, London, pp 3–22
Erecinska M, Silver IA (1989) ATP and brain function. J Cereb Blood Flow Metab 9:2–19
Benington JH, Heller C (1995) Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol 45:347–360
Lydic R, Baghdoyan HA, Hibbard L, Bonyak EV, De Joseph MR, Hawkins RA (1991) Regional brain glucose metabolism is altered during rapid eye movements in cat: a preliminary study. J Comp Neurol 304:517–529
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anaesthetised albino rat. J Neurochem 28:897–916
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE (1979) Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18) 2-Fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 6:371–388
Mottin S, Laporte P, Jouvet M, Cespuglio R (1997) Determination of NADH in the rat brain during sleep-wake states with an optic fibre sensor and time-resolved fluorescence procedures. Neuroscience 79:683–693
Chahboune H, Mahdjoub R, Desgoutte P, Rousset C, Briguet A, Cespuglio R (2008) Effects of chloramphenicol on brain energy metabolism using 31P spectroscopy: influences on sleep-wake states in rat. J Neurochem 106:1552–62
Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R (2010) Sleep and brain energy levels: ATP changes during sleep. J Neurosci 30:9008–9016
Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD (2002) Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci 22:5581–5587
Morgenthaler FD, Lanz BR, Petit JM, Frenkel H, Magistretti PJ, Gruetter R (2009) Alteration of brain glycogen turnover in the conscious rat after 5 h of prolonged wakefulness. Neurochem Int 55:45–51
Netchiporouk LI, Shram NF, Jaffrezic-Renault N, Martelet C, Cespuglio R (1996) In vivo brain glucose measurements: differential normal pulse voltammetry with enzyme-modified carbon fiber microelectrodes. Anal Chem 68:4358–4364
Shram NF, Netchiporouk LI, Martelet C, Jaffrezic-Renault N, Cespuglio R (1997) Brain glucose: voltammetric determination in normal and hyperglycaemic rats using a glucose microsensor. Neuroreport 8:1109–1112
Shram NF, Netchiporouk LI, Martelet C, Jaffrezic-Renault N, Bonnet C, Cespuglio R (1998) In vivo voltammetric detection of rat brain lactate with carbon fiber microelectrodes coated with lactate oxidase. Anal Chem 13:2618–2622
Soldatkin O, Schuvailo O, Marinesco S, Cespuglio R, Soldatkin A (2009) Microbiosensor based on glucose oxidase and hexokinase co-immobilised on platinum microelectrode for selective ATP detection. Talanta 78:1023–1028
Netchiporouk L, Shram N, Cespuglio R (2001) Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur J Neurosci 13:1429–1434
Shram N, Netchiporouk L, Cespuglio R (2002) Lactate in the brain of the freely moving rat: voltammetric monitoring of the changes related to the sleep-wake states. Eur J Neurosci 16:461–466
Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr (1992) Insulin in the brain: a hormonal regulator of energy balance. Endocrinol Rev 13:387–414
Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhof F (2001) Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. FASEB J 15:1270–1272
Burlet S, Leger L, Cespuglio R (1999) Nitric oxide and sleep in the rat: a puzzling relationship. Neuroscience 92:627–639
Acknowledgements
This work was supported by the University of Lyon and the Neuroscience Center of Lyon. The English form was checked by English Manager Sciences Editing.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Cespuglio, R., Netchiporouk, L., Shram, N. (2013). Glucose and Lactate Monitoring Across the Rat Sleep–Wake Cycle. In: Marinesco, S., Dale, N. (eds) Microelectrode Biosensors. Neuromethods, vol 80. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-370-1_11
Download citation
DOI: https://doi.org/10.1007/978-1-62703-370-1_11
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-369-5
Online ISBN: 978-1-62703-370-1
eBook Packages: Springer Protocols