Abstract
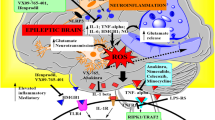
Seizures and epilepsy are associated with a neuroinflammatory process. Proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, are acutely expressed after seizures in experimental models and, in some cases, still overexpressed later during the chronic stage. Increased levels of these cytokines have been found in the serum from epileptic patients. Pharmacological approaches in rodents support that neuroinflammation is involved in modulating seizure susceptibility, neuronal damage, and other neurological disorders associated with epilepsy. This chapter review studies suggesting that proinflammatory cytokine systems are potential therapeutic targets for the pharmacological treatment of epilepsy.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ramesh G, MacLean AG, Philipp MT (2013) Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm 2013:480739
Kronfol Z, Remick DG (2000) Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry 157:683–694
Nathan C, Sporn M (1991) Cytokines in context. J Cell Biol 113:981–986
Minami M, Kuraishi Y, Yamaguchi T et al (1990) Convulsants induce interleukin-1 beta messenger RNA in rat brain. Biochem Biophys Res Commun 171:832–837
Minami M, Kuraishi Y, Satoh M (1991) Effects of kainic acid on messenger RNA levels of IL-1 beta, IL-6, TNF alpha and LIF in the rat brain. Biochem Biophys Res Commun 176:593–598
De Bock F, Dornand J, Rondouin G (1996) Release of TNF alpha in the rat hippocampus following epileptic seizures and excitotoxic neuronal damage. Neuroreport 7:1125–1129
Eriksson C, Winblad B, Schultzberg M (1998) Kainic acid induced expression of interleukin-1 receptor antagonist mRNA in the rat brain. Brain Res Mol Brain Res 58:195–208
Vezzani A, Conti M, De Luigi A et al (1999) Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci 19:5054–5065
De Simoni MG, Perego C, Ravizza T et al (2000) Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci 12:2623–2633
Plata-Salamán CR, Ilyin SE, Turrin NP et al (2000) Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res 75:248–258
O’Connor JJ, Coogan AN (1999) Actions of the pro-inflammatory cytokine IL-1 beta on central synaptic transmission. Exp Physiol 84:601–614
Dripps DJ, Brandhuber BJ, Thompson RC et al (1991) Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem 266:10331–10336
Dinarello CA (1994) The interleukin-1 family: 10 years of discovery. FASEB J 8:1314–1325
Loscher C (2003) Interleukin-1 receptor antagonist exerts agonist activity in the hippocampus independent of the interleukin-1 type I receptor. J Neuroimmunol 137:117–124
Ravizza T, Vezzani A (2006) Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience 137:301–308
Ravizza T, Gagliardi B, Noé F et al (2008) Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 29:142–160
Kołosowska K, Maciejak P, Szyndler J et al (2014) The role of interleukin-1β in the pentylenetetrazole-induced kindling of seizures, in the rat hippocampus. Eur J Pharmacol 731:31–37
Rizzi M, Perego C, Aliprandi M et al (2003) Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis 14:494–503
Ravizza T, Rizzi M, Perego C et al (2005) Inflammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia 46(Suppl 5):113–117
Järvelä JT, Lopez-Picon FR, Plysjuk A et al (2011) Temporal profiles of age-dependent changes in cytokine mRNA expression and glial cell activation after status epilepticus in postnatal rat hippocampus. J Neuroinflammation 8:29
Omran A, Peng J, Zhang C et al (2012) Interleukin-1β and microRNA-146a in an immature rat model and children with mesial temporal lobe epilepsy. Epilepsia 53:1215–1224
Álvarez-Croda DM, Santiago-García J, Medel-Matus JS, et al (2016) Hippocampal distribution of IL-1beta and IL-1RI following lithium-pilocarpine-induced status epilepticus in the developing rat. An Acad Bras Cienc 88 Suppl 1:653–63
Heida JG, Pittman QJ (2005) Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia 46:1906–1913
Dubé CM, Ravizza T, Hamamura M et al (2010) Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci 30:7484–7494
Bauer S, Cepok S, Todorova-Rudolph A et al (2009) Etiology and site of temporal lobe epilepsy influence postictal cytokine release. Epilepsy Res 86:82–88
Choi J, Min HJ, Shin J-S (2011) Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation 8:135
Nowak M, Bauer S, Haag A et al (2011) Interictal alterations of cytokines and leukocytes in patients with active epilepsy. Brain Behav Immun 25:423–428
Lehtimäki KA, Keränen T, Palmio J et al (2007) Increased plasma levels of cytokines after seizures in localization-related epilepsy. Acta Neurol Scand 116:226–230
Quirico-Santos T, Meira ID, Gomes AC et al (2013) Resection of the epileptogenic lesion abolishes seizures and reduces inflammatory cytokines of patients with temporal lobe epilepsy. J Neuroimmunol 254:125–130
Yu N, Di Q, Hu Y et al (2012) A meta-analysis of pro-inflammatory cytokines in the plasma of epileptic patients with recent seizure. Neurosci Lett 514:110–115
Uludag IF, Duksal T, Tiftikcioglu BI et al (2015) IL-1β, IL-6 and IL1Ra levels in temporal lobe epilepsy. Seizure 26:22–25
Lorigados Pedre L, Morales Chacón LM, Orozco Suárez S et al (2013) Inflammatory mediators in epilepsy. Curr Pharm Des 19:6766–6772
Ravizza T, Noé F, Zardoni D et al (2008) Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis 31:327–333
Ravizza T, Boer K, Redeker S et al (2006) The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiol Dis 24:128–143
Li G, Bauer S, Nowak M et al (2011) Cytokines and epilepsy. Seizure 20:249–256
Merrill JE, Benveniste EN (1996) Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 19:331–338
Maher CO, Anderson RE, Martin HS et al (2003) Interleukin-1beta and adverse effects on cerebral blood flow during long-term global hypoperfusion. J Neurosurg 99:907–912
Vezzani A, Moneta D, Richichi C et al (2002) Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia 43(Suppl 5):30–35
Chiavegato A, Zurolo E, Losi G et al (2014) The inflammatory molecules IL-1β and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front Cell Neurosci 8:155
Marchi N, Fan Q, Ghosh C et al (2009) Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis 33:171–181
Vezzani A, Moneta D, Conti M et al (2000) Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A 97:11534–11539
Galic MA, Riazi K, Heida JG et al (2008) Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci 28:6904–6913
Dubé C, Vezzani A, Behrens M et al (2005) Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol 57:152–155
Kwon YS, Pineda E, Auvin S et al (2013) Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation 10:30
Bar-Klein G, Cacheaux LP, Kamintsky L et al (2014) Losartan prevents acquired epilepsy via TGF-β signaling suppression. Ann Neurol 75:864–875
Gómez CD, Buijs RM, Sitges M (2014) The anti-seizure drugs vinpocetine and carbamazepine, but not valproic acid, reduce inflammatory IL-1β and TNF-α expression in rat hippocampus. J Neurochem 130:770–779
Sitges M, Gómez CD, Aldana BI (2014) Sertraline reduces IL-1β and TNF-α mRNA expression and overcomes their rise induced by seizures in the rat hippocampus. PLoS One 9:e111665
Chiu KM, Wu CC, Wang MJ et al (2015) Protective effects of bupivacaine against kainic acid-induced seizure and neuronal cell death in the rat hippocampus. Biol Pharm Bull 38:522–530
Noe FM, Polascheck N, Frigerio F et al (2013) Pharmacological blockade of IL-1β/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis 59:183–193
Sankar R, Shin DH, Liu H et al (1998) Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci 18:8382–8393
Niquet J, Auvin S, Archie M et al (2007) Status epilepticus triggers caspase-3 activation and necrosis in the immature rat brain. Epilepsia 48:1203–1206
Lopez-Meraz M-L, Wasterlain CG, Rocha LL et al (2010) Vulnerability of postnatal hippocampal neurons to seizures varies regionally with their maturational stage. Neurobiol Dis 37:394–402
Medel-Matus J-S, Álvarez-Croda D-M, Martínez-Quiroz J et al (2014) IL-1β increases necrotic neuronal cell death in the developing rat hippocampus after status epilepticus by activating type I IL-1 receptor (IL-1RI). Int J Dev Neurosci 38:232–240
Auvin S, Shin D, Mazarati A et al (2007) Inflammation exacerbates seizure-induced injury in the immature brain. Epilepsia 48(Suppl 5):27–34
Lin TY, Lu CW, Wang SJ et al (2015) Protective effect of hispidulin on kainic acid-induced seizures and neurotoxicity in rats. Eur J Pharmacol 755:6–15
Ma XC, Gottschall PE, Chen LT et al (2011) Role and mechanisms of interleukin-1 in the modulation of neurotoxicity. Neuroimmunomodulation 10:199–207
Yan X, Yadav R, Gao M et al (2014) Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia 62:1093–1109
Wang S, Cheng Q, Malik S et al (2000) Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther 292:497–504
Viviani B, Bartesaghi S, Gardoni F et al (2003) Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 23:8692–8700
Miller LG, Galpern WR, Dunlap K et al (1991) Interleukin-1 augments gamma-aminobutyric acid A receptor function in brain. Mol Pharmacol 39:105–108
Zhu G, Okada M, Yoshida S et al (2006) Effects of interleukin-1beta on hippocampal glutamate and GABA releases associated with Ca2+-induced Ca2+ releasing systems. Epilepsy Res 71:107–116
Takeda M, Kitagawa J, Takahashi M et al (2008) Activation of interleukin-1beta receptor suppresses the voltage-gated potassium currents in the small-diameter trigeminal ganglion neurons following peripheral inflammation. Pain 139:594–602
Meini A, Benocci A, Frosini M et al (2000) Nitric oxide modulation of interleukin-1[beta]-evoked intracellular Ca2+ release in human astrocytoma U-373 MG cells and brain striatal slices. J Neurosci 20:8980–8986
Roseti C, van Vliet EA, Cifelli P et al (2015) GABAA currents are decreased by IL-1β in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiol Dis 82:311–320
Helmstaedter C, Aldenkamp AP, Baker GA et al (2014) Disentangling the relationship between epilepsy and its behavioral comorbidities—the need for prospective studies in new-onset epilepsies. Epilepsy Behav 31:43–47
Zunszain PA, Hepgul N, Pariante CM (2013) Inflammation and depression. Curr Top Behav Neurosci 14:135–151
Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741
Mazarati AM, Shin D, Kwon YS et al (2009) Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol Dis 34:457–461
Pineda E, Shin D, Sankar R et al (2010) Comorbidity between epilepsy and depression: experimental evidence for the involvement of serotonergic, glucocorticoid, and neuroinflammatory mechanisms. Epilepsia 51(Suppl 3):110–114
Dowlati Y, Herrmann N, Swardfager W et al (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457
Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186
Khandaker GM, Pearson RM, Zammit S et al (2014) Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71:1121–1128
Levine J, Barak Y, Chengappa KN et al (1999) Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology 40:171–176
Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M et al (2011) Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 16:751–762
Krishnadas R, Cavanagh J (2012) Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry 83:495–502
Berthold-Losleben M, Himmerich H (2008) The TNF-alpha system: functional aspects in depression, narcolepsy and psychopharmacology. Curr Neuropharmacol 6:193–202
Doczy EJ, Seroogy K, Harrison CR et al (2009) Hypothalamo-pituitary-adrenocortical axis, glucocorticoids, and neurologic disease. Immunol Allergy Clin North Am 29:265–284
Cowen PJ (2002) Cortisol, serotonin and depression: all stressed out? Br J Psychiatry 180:99–100
Young EA, Haskett RF, Grunhaus L et al (1994) Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Arch Gen Psychiatry 51:701–707
Zhu C-B, Blakely RD, Hewlett WA (2006) The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 31:2121–2131
Hou R, Baldwin DS (2012) A neuroimmunological perspective on anxiety disorders. Hum Psychopharmacol 27:6–14
Gill J, Vythilingam M, Page GG (2008) Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress 21:530–539
Rohleder N, Joksimovic L, Wolf JM et al (2004) Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry 55:745–751
Von Känel R, Begré S, Abbas CC et al (2010) Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation 17:39–46
Hoge EA, Brandstetter K, Moshier S et al (2009) Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 26:447–455
Leonard BE, Myint A (2009) The psychoneuroimmunology of depression. Hum Psychopharmacol 24:165–175
Wei H, Alberts I, Li X (2013) Brain IL-6 and autism. Neuroscience 252:320–325
Smith SEP, Li J, Garbett K et al (2007) Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27:10695–10702
Pineda E, Shin D, You SJ et al (2013) Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann Neurol 74:11–19
Washington J, Kumar U, Medel-Matus J-S et al (2015) Cytokine-dependent bidirectional connection between impaired social behavior and susceptibility to seizures associated with maternal immune activation in mice. Epilepsy Behav 50:40–45
Heo Y, Zhang Y, Gao D et al (2011) Aberrant immune responses in a mouse with behavioral disorders. PLoS One 6:e20912
Vargas DL, Nascimbene C, Krishnan C et al (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57:67–81
Li X, Chauhan A, Sheikh AM et al (2009) Elevated immune response in the brain of autistic patients. J Neuroimmunol 207:111–116
Ashwood P, Krakowiak P, Hertz-Picciotto I et al (2011) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25:40–45
Xu N, Li X, Zhong Y (2015) Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediators Inflamm 2015:531518
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–86.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Appendix 1: Intracerebroventricular (i.c.v) Injection of Cytokines in 14-Day-Old Rat Pups
1.1 Materials
Anesthesia system, isoflurane anesthesia system, hair clippers, scalpel, forceps, fine scissors, 25-gauge needle, stereotaxic frame adapted for neonatal rodents, heating blanket, handheld drill for skull openings, microinfusion pump, bone wax, sterile suture, and dry glass bead sterilizer.
1.2 Solutions
Picric acid saturated solution, sterile saline.
1.3 Protocol
-
1.
Anesthetize the rat with isoflurane (3 %, for induction) and shave the top of the head.
-
2.
Place the anesthetized rat in a stereotaxic frame adapted for neonatal rodents and maintain the rat’s body temperature with a heating blanket. Anesthetized rats with isoflurane (1.5–2 %, for maintenance) during the surgery.
Note: Surgery must be performed in aseptic conditions; surgical instruments must be sterilized.
-
3.
Clean the skin with iodine solution to reduce the risk of contamination.
-
4.
Make a small incision in the skin to expose the skull.
-
5.
Make a small burr hole at the following coordinates: −4.0 mm posterior to bregma, −1.6 mm lateral to the midline (right side).
-
6.
Insert a 25-gauge needle (0.5 mm) into the hole and lower it until it is at the depth of 3.5 mm below the surface of the skull.
-
7.
Infuse drug over a period of 5 min (0.2 μl/min) using a microinfusion pump.
Note: Prepare stock solution of lyophilized drug by dissolving them in 1 μl of sterile saline solution.
-
8.
Seal the hole with bone wax and close the skin with a single suture.
-
9.
Apply the saturated picric acid solution to the wound to prevent maternal cannibalism.
-
10.
After recovery, the pup is transferred to its mother.
Appendix 2: Determination of IL-1β and IL-1RI mRNA Expression by qRT-PCR
2.1 Materials
mirVanaTM PARISTM kit (AM1556, Applied Biosystems, USA), agarose gel for electrophoresis, M-MLV reverse transcriptase (Invitrogen, USA), cDNA, primers (forward and reverse), Maxima SYBR Green/ROX qPCR Master Mix (Fermentas, Thermo Scientific, USA), nuclease-free water, spectrophotometer, electrophoresis system, and thermal cycler.
2.2 Protocol
-
1.
Isolate RNA from brain samples with mirVanaTM PARISTM kit (AM1556) following the manufacturer’s recommendations (Applied Biosystems, USA).
-
2.
Determine RNA concentration with a spectrophotometer by measuring the absorbance at 260 nm.
-
3.
Assess RNA integrity by agarose gel electrophoresis.
-
4.
Perform reverse transcription reactions with 1 μg of total RNA in a final volume of 20 μl, using M-MLV reverse transcriptase (Invitrogen, USA), according to the manufacturer’s recommendations.
-
5.
Perform real-time PCR reactions in triplicate as follows: each sample containing 1 μl of cDNA, 5 pmoles of each primer (forward and reverse), 10 μl of Maxima SYBR Green/ROX qPCR Master Mix (Fermentas, Thermo Scientific, USA), and nuclease-free water up to 20 μl.
Note: Include triplicates for each sample containing primers for β-actin to normalize the data.
-
6.
Run reactions in a thermal cycler, according to the following protocol: 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 30 s at 95 °C, and 1 min at 60 °C, followed by a melting curve analysis.
Note: Primers were designed using the Primer 3 software [96], and sequences are shown in Table 1.
-
7.
Calculate amplification efficiencies by using lineal regression and determine changes in relative gene expression using β-actin as internal control.
Appendix 3: Localization of IL-1β and IL-1RI by Immunohistochemistry
3.1 Materials
Peristaltic pump, microtome, humidity chamber, histology jars, hydrophobic pen, aqueous mounting media, microwave, timer, and light microscope.
3.2 Solutions
4 % phosphate-buffered paraformaldehyde, 0.1 M phosphate buffer (0.1 M PB), saline solution, 10 mM citrate solution (pH 6.0), ethanol solutions: 70, 80, 95, and 100 %, blocking buffer (0.5 % goat serum in 0.1 M PB).
3.3 Protocol
3.3.1 Tissue Processing
-
1.
Anesthetize rats with an overdose of pentobarbital.
-
2.
Perform transcardiac perfusion with saline solution followed by 4 % phosphate-buffered (PB) paraformaldehyde.
-
3.
Keep brain in situ at 4 °C overnight.
-
4.
After removal of the brain, dehydrate in graded series of ethanol solutions (70 %, 8 h; 80 %, 2 × 3 h; 95 %, 2 × 1.5 h; 100 %, 2 × 1 h) and xylene (2 × 40 min) and then embed it in paraffin.
-
5.
Section brains into 10-μm-thick coronal sections at the level of dorsal hippocampus and mount sections on gelatin-coated glass slides; keep slides at room temperature.
-
6.
Deparaffinize (in xylene, 2 × 10 min) and rehydrate sections in a graded series of ethanol (100 %, 2 × 10 min; 95 %, 2 × 5 min; and 70 %, 1 × 5 min) and dH2O (2 × 10 min) with a final wash in 0.1 M PB (1 × 10 min).
3.3.2 Immunodetection of IL-1β and IL-1RI
-
1.
Perform antigen retrieval procedure by heating brain sections in a 10 mM citrate solution (pH 6.0) using a microwave oven [2 min at high power and 10 min at power 20]; then allow the slides to slowly cool to room temperature for 45 min.
-
2.
Rinse slides in dH2O (1 × 10 min), wash them in 0.1 M PB (2 × 10 min), and incubate in a blocking buffer (0.5 % goat serum in 0.1 M PB) at room temperature for 1 h.
-
3.
Incubate slides overnight at 4 °C in a humidified chamber with primary antibody [rabbit polyclonal anti-IL-1β (ab9787, Abcam) diluted 1:500 or rabbit polyclonal anti-IL-1RI (sc 25775, Santa Cruz) diluted 1:200 with blocking buffer].
-
4.
Wash sections in 0.1 M PB (3 × 10 min) and then expose them to biotinylated goat anti-rabbit (BA 1000, Vector Labs) diluted 1:1000 with the blocking solution for 2 h at room temperature.
-
5.
Wash sections in 0.1 M PB (3 × 10 min) and then incubate with ABC-peroxidase complex 1:1000 (Vectastain ABC kit elite DK-6100 standard, Vector Labs) during 90 min.
-
6.
Wash sections in 0.1 M PB (3 × 10 min) and reveal staining after incubation with AB peroxidase substrate kit, 3,3′-diaminobenzidine (SK-4100, Vector Labs).
-
7.
Wash sections in 0.1 M PB (3 × 10 min), dehydrate it in graded series of ethanol solutions (70 %, 1 min; 95 %, 1 min; 100 %, 1 min) and xylene (20 dips), and then mount with nonaqueous mounting medium.
-
8.
Examine brain sections with a light microscope.
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
López-Meraz, ML., Medel-Matus, JS., Niquet, J. (2016). Inflammatory Cytokines as Targets for Epilepsy Drug Therapy. In: Talevi, A., Rocha, L. (eds) Antiepileptic Drug Discovery. Methods in Pharmacology and Toxicology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-6355-3_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6355-3_1
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-6353-9
Online ISBN: 978-1-4939-6355-3
eBook Packages: Springer Protocols