Abstract
The twentieth century was a landmark period in the history of cancer therapies; a time in which conventional cancer treatments such as surgery resection, radiotherapy and chemotherapy made tremendous advances and gave birth to strategies focused on greater selective targeting. Rational drug design allied to rational drug delivery, being led by novel small molecule anti-cancer compounds and monoclonal antibodies. Such approaches prolonged survival time, often without the horrific side effects of previous therapies, but rarely prevented ultimate disease relapse. Selective targeting of membrane transporters and receptors using prodrugs, polymer-cancer drug conjugates and antibody-toxin combinations has contributed to redefine the roadmap of cancer cell targeting. Current pharmacotherapies are still far from consistently delivering cures or sustained remissions. This chapter describes some of the historical events that brought about current cancer targeting strategies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
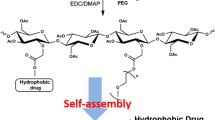
Similar content being viewed by others
References
DeVita VT, Chu E (2008) A history of cancer chemotherapy. Cancer Res 68(21):8643–8653. doi:10.1158/0008-5472.can-07-6611
Strebhardt K, Ullrich A (2008) Paul ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer 8(6):473–480. doi:nrc2394 [pii] 10.1038/nrc2394
American Cancer Society website (2009) The history of cancer. http://www.cancer.org/acs/groups/cid/documents/webcontent/002048-pdf.pdf
Kufe DW, Holland JF, Frei E, Kufe D ((2003) Cancer medicine 6 review: a companion to holland-frei cancer medicine-6. B.C. Decker, Hamilton, Ont.; London
Ariel IM (1991) The role of surgery in the treatment of breast cancer: historical review and current status. Springer-Verlag, High risk breast cancer therapy
Cancer Medicine, KD. RC Bast, Pollock RE, et al, Editor 2000, Hamilton (ON): BC Decker Henderson BE, BL., Ross RK (2000). Chapter 13: Hormones and the Etiology of Cancer. K. D. RC Bast, Pollock RE, et al. Ontario, Hamilton (ON): BC Decker.
Denoix PF (1946) Enquete permanent dans les centres anticancereaux. Bull Inst Nat Hyg 1:70–75
Sabatine M (2007) Sabatine’s essentials of internal medicine. Philadelphia Lippincott Williams & Wilkins
Sikora E (1990) Treatment of cancer, 2nd edn. London, Chapman and Hall Medical
Begg AC, Stewart FA, Vens C (2011) Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 11(4):239–253
Meyn RE, Withers HR (1980) Radiation biology in cancer research. Raven, New York
Curie P, Curie M, Bemont G (1898) Sur une nouvelle substance fortement radio-active contenue dans la pechblende. CRT 127:1215–1217
Lawrence JH, Aebersold PC, Lawrence EO (1936) Comparative effects of x-rays and neutrons on normal and tumor tissue. Proc Natl Acad Sci U S A 22(9):543–557. doi:10.2307/86432
Puck TT, Marcus PI (1956) Action of x-rays on mammalian cells. J Exp Med 103(5):653–666
Prise KM, Schettino G, Folkard M, Held KD (2005) New insights on cell death from radiation exposure. Lancet Oncol 6(7):520–528. doi:10.1016/s1470-2045(05)70246-1
Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J (1996) Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst 88(13):918–922
Bentzen SM, Overgaard M, Thames HD (1989) Fractionation sensitivity of a functional endpoint - impaired shoulder movement after post-mastectomy radiotherapy. Int J Radiat Oncol Biol Phys 17(3):531–537
Rubin P, Johnston CJ, Williams JP, Mcdonald S, Finkelstein JN (1995) A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 33(1):99–109. doi:10.1016/0360-3016(95)00095-G
Jeggo P, Lavin MF (2009) Cellular radiosensitivity: how much better do we understand it? Int J Radiat Biol 85(12):1061–1081. doi:10.3109/09553000903261263
Delaney G, Jacob S, Featherstone C, Barton M (2005) The role of radiotherapy in cancer treatment. Cancer 104(6):1129–1137. doi:10.1002/cncr.21324
Dexheimer TS (2013) DNA repair pathways and mechanisms. In: Mathews LA, Cabarcas SM, Hurt EM (eds) DNA repair of cancer stem cells. Springer, Netherlands, pp 19–32. doi:10.1007/978-94-007-4590-2_2
Bentzen SM (2006) Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 6(9):702–713. doi:10.1038/Nrc1950
Charles A, Ramani A (2011) Phytochemical screening and antimicrobial resistance of alseodaphne semecarpifolia nees. J Chem Pharm Res 3(5):205–211
Goodman L (1984) Nitrogen mustard therapy: use of methyl-bis(beta-chloroethyl)amine hydrochloride and tris(beta-chloroethyl)amine hydrochloride for hodgkin´s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. JAMA 251(17):2255–2261. doi:10.1001/jama.1984.03340410063036
Farber S, Diamond LK, Mercer RD, Sylvester RF, Wolff JA (1948) Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid (aminopterin). N Engl J Med 238(23):787–793. doi:10.1056/NEJM194806032382301
Li MC, Hertz R, Bergenstal DM (1958) Therapy of choriocarcinoma and related trophoblastic tumors with folic acid and purine antagonists. N Engl J Med 259(2):66–74. doi:10.1056/NEJM195807102590204
Brockman RW (1963) Mechanisms of resistance to anticancer agents. Adv Cancer Res 7:129–234. doi:10.1016/S0065-230x(08)60983-5
Al-Lazikani B, Banerji U, Workman P (2012) Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol 30(7):679–692
Chabner BA, Roberts TG (2005) Chemotherapy and the war on cancer. Nat Rev Cancer 5(1):65–72
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346(2):85–91. doi:10.1056/NEJMoa003034
Bodurka DC, Levenback C, Wolf JK, Gano J, Wharton JT, Kavanagh JJ, Gershenson DM (2003) Phase ii trial of irinotecan in patients with metastatic epithelial ovarian cancer or peritoneal cancer. J Clin Oncol 21(2):291–297. doi:10.1200/jco.2003.02.091
Workman P (2002) The impact of genomic and proteomic technologies on the development of new cancer drugs. Ann Oncol 13(suppl 4):115–124
Heppner GH (1984) Tumor heterogeneity. Cancer Res 44(6):2259–2265
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70, http://dx.doi.org/10.1016/S0092-8674(00)81683-9
Alaimo PJ, Shogren-Knaak MA, Shokat KM (2001) Chemical genetic approaches for the elucidation of signaling pathways. Curr Opin Chem Biol 5(4):360–367. doi:10.1016/S1367-5931(00)00215-5
Scott AM, Allison JP, Wolchok JD (2012) Monoclonal antibodies in cancer therapy. Cancer Immun 12:14
Wiseman GA, White CA, Stabin M, Dunn WL, Erwin W, Dahlbom M, Raubitschek A, Karvelis K, Schultheiss T, Witzig TE, Belanger R, Spies S, Silverman DHS, Berlfein JR, Ding E, Grillo-López AJ (2000) Phase i/ii 90y-zevalin (yttrium-90 ibritumomab tiuxetan, idec-y2b8) radioimmunotherapy dosimetry results in relapsed or refractory non-hodgkin’s lymphoma. Eur J Nucl Med 27(7):766–777. doi:10.1007/s002590000276
Chari RVJ, Martell BA, Gross JL, Cook SB, Shah SA, Blättler WA, McKenzie SJ, Goldmacher VS (1992) Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res 52(1):127–131
Remillard S, Rebhun LI, Howie GA, Kupchan SM (1975) Antimitotic activity of the potent tumor inhibitor maytansine. Science (New York, NY) 189(4207):1002–1005
Oldham RK, Dillman RO (2008) Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol 26(11):1774–1777. doi:10.1200/Jco.2007.15.7438
Dillman R (2003) Monoclonal antibody therapy. Principles of cancer biotherapy, 4th edn. Kluwer, Norwell, MA
Atkins JH, Gershell LJ (2002) Selective anticancer drugs. Nat Rev Drug Discov 1(7):491–492
Arteaga CL, Baselga J (2004) Tyrosine kinase inhibitors: why does the current process of clinical development not apply to them? Cancer Cell 5(6):525–531. doi:10.1016/j.ccr.2004.05.028
Ciardiello F, Vita F (2005) Epidermal growth factor receptor (egfr) inhibitors in cancer therapy. In: Herrling P, Matter A, Schultz R (eds) Advances in targeted cancer therapy, vol 63. Progress in drug research. Birkhäuser, Basel, pp 93–115. doi:10.1007/3-7643-7414-4_5
Salomon DS, Brandt R, Ciardiello F, Normanno N (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19(3):183–232. doi:10.1016/1040-8428(94)00144-i
Xu H, Yu YJ, Marciniak D, Rishi AK, Sarkar FH, Kucuk O, Majumdar APN (2005) Epidermal growth factor receptor (egfr)-related protein inhibits multiple members of the egfr family in colon and breast cancer cells. Mol Cancer Ther 4(3):435–442
Perez-Soler R (2004) Her1/egfr targeting: refining the strategy. Oncologist 9(1):58–67. doi:10.1634/theoncologist.9-1-58
Bange J, Zwick E, Ullrich A (2001) Molecular targets for breast cancer therapy and prevention. Nat Med 7(5):548–552. doi:10.1038/87872
Mackey JR, Clemons M, Cote MA, Delgado D, Dent S, Paterson A, Provencher L, Sawyer MB, Verma S (2008) Cardiac management during adjuvant trastuzumab therapy: recommendations of the canadian trastuzumab working group. Curr Oncol 15(1):24–35. doi:10.3747/Co.2008.199
Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, von Pawel J, Pluzanska A, Gatzemeier M, Grous J, Ochs JS, Averbuch SD, Wolf MK, Rennie P, Fandi A, Johnson DH (2004) Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase iii trial-intact1. J Clin Oncol 22(5):777–784. doi:10.1200/Jco.2004.08.001
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350(21):2129–2139. doi:10.1056/NEJMoa040938
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M (2004) Egfr mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304(5676):1497–1500. doi:10.1126/science.1099314
Algire GH, Legallais FY (1951) Vascular reactions of normal and malignant tissues invivo.1. The effect of peripheral hypotension on transplanted tumors. J Natl Cancer Inst 12(2):399
Ferrara N, Gerber HP, LeCouter J (2003) The biology of vegf and its receptors. Nat Med 9(6):669–676. doi:10.1038/Nm0603-669
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor-cells secrete a vascular-permeability factor that promotes accumulation of ascites-fluid. Science 219(4587):983–985. doi:10.1126/science.6823562
Ferrara N, DavisSmyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18(1):4–25. doi:10.1210/Er.18.1.4
Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK (2004) Direct evidence that the vegf-specific antibody bevacizumab has antivascular effects in human rectal cancer (vol 10, pg 145, 2004). Nat Med 10(6):649–649. doi:10.1038/Nm0604-649c
Singh M, Ferrara N (2012) Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nat Biotechnol 30(7):648–657
Kerbel R, Folkman J (2002) Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2(10):727–739. doi:10.1038/Nrc905
Ferrara N, Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature 438(7070):967–974. doi:10.1038/Nature04483
Gerber HP, Dixit V, Ferrara N (1998) Vascular endothelial growth factor induces expression of the antiapoptotic proteins bcl-2 and a1 in vascular endothelial cells. J Biol Chem 273(21):13313–13316. doi:10.1074/jbc.273.21.13313
Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N (1999) Vegf is required for growth and survival in neonatal mice. Development 126(6):1149–1159
Reed JC (2005) Apoptosis-based therapies for cancer. Clin Cancer Res 11(24):9175s–9175s
Hu W, Kavanagh JJ (2003) Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 4(12):721–729. doi:S1470204503012774 [pii]
Los M, Burek CJ, Stroh C, Benedyk K, Hug H, Mackiewicz A (2003) Anticancer drugs of tomorrow: apoptotic pathways as targets for drug design. Drug Discov Today 8(2):67–77. doi:Pii S1359-6446(02)02563-1, Doi 10.1016/S1359-6446(02)02563-1
Nakamoto T, Inagawa H, Takagi K, Soma GI (2000) A new method of antitumor therapy with a high dose of tnf perfusion for unresectable liver tumors. Anticancer Res 20(6A):4087–4096
Johnstone RW, Frew AJ, Smyth MJ (2008) The trail apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer 8(10):782–798
Xiang H, Fox JA, Totpal K, Aikawa M, Dupree K, Sinicropi D, Lowe J, Escandón E (2002) Enhanced tumor killing by apo2l/trail and cpt-11 co-treatment is associated with p21 cleavage and differential regulation of apo2l/trail ligand and its receptors. Oncogene 21(22):3611–3619
Jo M, Kim T-H, Seol D-W, Esplen JE, Dorko K, Billiar TR, Strom SC (2000) Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med 6(5):564–567
Srivastava RK, Mi QS, Hardwick JM, Longo DL (1999) Deletion of the loop region of bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci U S A 96(7):3775–3780. doi:10.1073/pnas.96.7.3775
Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, Toge T (2002) Current status of the molecular mechanisms of anticancer drug-induced apoptosis. Cancer Chemother Pharmacol 50(5):343–352. doi:10.1007/s00280-002-0522-7
LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien CS, Adams J, Gupta D, Richardson PG, Munshi NC, Anderson KC (2002) Proteasome inhibitor ps-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res 62(17):4996–5000
Heppner G, Miller B (1983) Tumor heterogeneity: biological implications and therapeutic consequences. Cancer Metast Rev 2(1):5–23. doi:10.1007/bf00046903
de Bono JS, Ashworth A (2010) Translating cancer research into targeted therapeutics. Nature 467(7315):543–549. doi:10.1038/Nature09339
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316(8):1324–1331. doi:10.1016/j.yexcr.2010.02.045
Li XQ, Ma QY, Xu QH, Duan WX, Lei JJ, Wu EX (2012) Targeting the cancer-stroma interaction: a potential approach for pancreatic cancer treatment. Curr Pharm Design 18(17):2404–2415
Jaffee EM, Hruban RH, Canto M, Kern SE (2002) Focus on pancreas cancer. Cancer Cell 2(1):25–28. doi:10.1016/S1535-6108(02)00093-4
Yang WQ, Zhang Y (2012) Rnai-mediated gene silencing in cancer therapy. Expert Opin Biol Ther 12(11):1495–1504. doi:10.1517/14712598.2012.712107
Davidson BL, McCray PB (2011) Current prospects for rna interference-based therapies. Nat Rev Genet 12(5):329–340. doi:10.1038/Nrg2968
Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, Giangrande PH (2009) Systemic administration of optimized aptamer-sirna chimeras promotes regression of psma-expressing tumors. Nat Biotechnol 27(9):839–U895. doi:10.1038/Nbt.1560
Devi GR (2006) Sirna-based approaches in cancer therapy. Cancer Gene Ther 13(9):819–829. doi:10.1038/sj.cgt.7700931
Nakanishi T (2007) Drug transporters as targets for cancer chemotherapy. Cancer Genomics Proteomics 4(3):241–254
Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Kamen BA (1992) Distribution of the folate receptor gp38 in normal and malignant cell lines and tissues. Cancer Res 52(12):3396–3401
Sudimack J, Lee RJ (2000) Targeted drug delivery via the folate receptor. Adv Drug Deliv Rev 41(2):147–162, http://dx.doi.org/10.1016/S0169-409X(99)00062-9
Gabizon A, Horowitz AT, Goren D, Tzemach D, Mandelbaum-Shavit F, Qazen MM, Zalipsky S (1999) Targeting folate receptor with folate linked to extremities of poly(ethylene glycol)-grafted liposomes: in vitro studies. Bioconjug Chem 10(2):289–298. doi:10.1021/bc9801124
Lee RJ, Low PS (1995) Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim Biophys Acta (BBA) – Biomembranes 1233(2):134–144, http://dx.doi.org/10.1016/0005-2736(94)00235-H
Tsuji A (1999) Tissue selective drug delivery utilizing carrier-mediated transport systems. J Control Release 62(1–2):239–244, http://dx.doi.org/10.1016/S0168-3659(99)00043-7
Knutter I, Rubio-Aliaga I, Boll M, Hause G, Daniel H, Neubert K, Brandsch M (2002) H+−peptide cotransport in the human bile duct epithelium cell line sk-cha-1. Am J Physiol Gastrointest Liver Physiol 283(1):G222–G229. doi:10.1152/ajpgi.00534.2001
Gonzalez DE, Covitz K-MY, Sadée W, Mrsny RJ (1998) An oligopeptide transporter is expressed at high levels in the pancreatic carcinoma cell lines aspc-1 and capan-2. Cancer Res 58(3):519–525
Mitsuoka K, Miyoshi S, Kato Y, Murakami Y, Utsumi R, Kubo Y, Noda A, Nakamura Y, Nishimura S, Tsuji A (2008) Cancer detection using a pet tracer, c-11-glycylsarcosine, targeted to h+/peptide transporter. J Nucl Med 49(4):615–622. doi:10.2967/jnumed.107.048231
Nakanishi T, Tamai I, Takaki A, Tsuji A (2000) Cancer cell-targeted drug delivery utilizing oligopeptide transport activity. Int J Cancer 88(2):274–280. doi:10.1002/1097-0215(20001015)88:2<274::Aid-Ijc20>3.0.Co;2–5
Feng Cao YG, Ping Q (2012) Advances in research of pept1-targeted prodrug. ÑÇÖÞÒ©ÎïÖƼÁ¿Æѧ 7(2) Asian Journal of Pharmaceutical Sciences: 110–110
Song X, Lorenzi PL, Landowski CP, Vig BS, Hilfinger JM, Amidon GL (2005) Amino acid ester prodrugs of the anticancer agent gemcitabine: synthesis, bioconversion, metabolic bioevasion, and hpept1-mediated transport. Mol Pharm 2(2):157–167. doi:10.1021/mp049888e
Tsume Y, Hilfinger JM, Amidon GL (2008) Enhanced cancer cell growth inhibition by dipeptide prodrugs of floxuridine: increased transporter affinity and metabolic stability. Mol Pharm 5(5):717–727. doi:10.1021/mp800008c
Lee VHL (2000) Membrane transporters. Eur J Pharm Sci 11 2(Suppl 2 11):S41–S411. doi:10.1016/s0928-0987(00)00163-9
Mrsny RJ (1998) Oligopeptide transporters as putative therapeutic targets for cancer cells - commentary. Pharmaceut Res 15(6):816–818. doi:10.1023/A:1011904027137
Duncan R (2006) Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 6(9):688–701
Folkman J, Long DM, Rosenbaum R (1967) Silicone rubber: a new diffusion property useful for general anesthesia. Rubb Chem Technol 40(3):928–931. doi:10.5254/1.3539107
Folkman J, Long DM (1964) The use of silicone rubber as a carrier for prolonged drug therapy. J Surg Res 4:139–142. doi:10.1016/s0022-4804(64)80040-8
Hoffman AS (2008) The origins and evolution of “controlled” drug delivery systems. J Control Release 132(3):153–163, http://dx.doi.org/10.1016/j.jconrel.2008.08.012
Ringsdorf H (1975) Structure and properties of pharmacologically active polymers. J Polym Sci: Polymer Symposia 51(1):135–153. doi:10.1002/polc.5070510111
Duncan R, Vicent MJ, Greco F, Nicholson RI (2005) Polymer–drug conjugates: towards a novel approach for the treatment of endrocine-related cancer. Endocr Relat Cancer 12(Suppl 1):S189–S199. doi:10.1677/erc.1.01045
Duncan R (1997) Polymer therapeutics for tumour specific delivery. Chem Ind-London 7:262–264
Maeda H, Ueda M, Morinaga T, Matsumoto T (1985) Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J Med Chem 28(4):455–461. doi:10.1021/jm00382a012
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46(12 Part 1):6387–6392
Maeda H, Matsumura Y (1989) Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev Ther Drug Carrier Syst 6(3):193–210
Chytil P, Etrych T, Konák C, Sírová M, Mrkvan T, Boucek J, Ríhová B, Ulbrich K (2008) New hpma copolymer-based drug carriers with covalently bound hydrophobic substituents for solid tumour targeting. J Control Release 127(2):121–130
Li C, Wallace S (2008) Polymer-drug conjugates: recent development in clinical oncology. Adv Drug Deliv Rev 60(8):886–898
Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, Doran J, Young AM, Burtles S, Kerr DJ, Committee ftCRCPIICT (2002) Hepatic drug targeting: phase i evaluation of polymer-bound doxorubicin. J Clin Oncol 20(6):1668–1676. doi:10.1200/jco.20.6.1668
Shashwat BNA, Rajesh P, Jayant K (2012) Poly(ethylene glycol)-prodrug conjugates: concept, design, and applications. J Drug Deliv 2012:17. doi:10.1155/2012/103973
Lindahl T, Barnes DE (2000) Repair of endogenous DNA damage. Cold Spring Harb Sym 65:127–133. doi:10.1101/sqb.2000.65.127
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Cravo, A.S., Mrsny, R.J. (2013). A Time Travel Journey Through Cancer Therapies. In: Bae, Y., Mrsny, R., Park, K. (eds) Cancer Targeted Drug Delivery. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7876-8_1
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7876-8_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7875-1
Online ISBN: 978-1-4614-7876-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)