Abstract
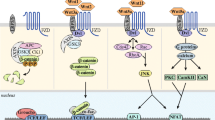
Multiple myeloma (MM) is a complex and still incurable disease which strongly relies on a network of humoral and cellular interactions within the human bone marrow milieu. The canonical Wnt/b-catenin and the alternative Wnt/RhoA-signaling pathways play important roles in the tropism between MM cells and BM microenvironment, and they have recently been implicated in MM pathogenesis and development of MM bone disease. However, their precise role in growth and survival of myeloma cells remains controversial and needs further investigation. We here summarize the most recent updates of the Wnt/β-catenin signaling pathway in myeloma, and discuss how its various components contribute to MM pathogenesis and related bone disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rajkumar SV, Kyle RA (2005) Multiple myeloma: diagnosis and treatment. Mayo Clin Proc 80:1371–1382
Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC (2004) Focus on multiple myeloma. Cancer Cell 6:439–444
Kuehl WM, Bergsagel PL (2002) Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer 2:175–187
Carrasco DR, Tonon G, Huang Y et al (2006) High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell 9:313–325
Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC (2007) Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 7:585–598
Podar K, Chauhan D, Anderson KC (2009) Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 23:10–24
Qiang YW, Endo Y, Rubin JS, Rudikoff S (2003) Wnt signaling in B-cell neoplasia. Oncogene 22:1536–1545
Fulciniti M, Tassone P, Hideshima T et al (2009) Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114:371–379
Giuliani N, Morandi F, Tagliaferri S et al (2007) Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res 67:7665–7674
Barker N, Clevers H (2006) Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov 5:997–1014
Wodarz A, Nusse R (1998) Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14:59–88
Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5:367–377
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
He X, Semenov M, Tamai K, Zeng X (2004) LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131:1663–1677
Wharton KA Jr (2003) Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol 253:1–17
Moon RT, Bowerman B, Boutros M, Perrimon N (2002) The promise and perils of Wnt signaling through beta-catenin. Science 296:1644–1646
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Willert K, Nusse R (1998) Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 8:95–102
Ben-Ze’ev A, Geiger B (1998) Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol 10:629–639
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R (1997) beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16:3797–3804
Molenaar M, van de Wetering M, Oosterwegel M et al (1996) XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86:391–399
Behrens J, von Kries JP, Kuhl M et al (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638–642
He TC, Sparks AB, Rago C et al (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Shtutman M, Zhurinsky J, Simcha I et al (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96:5522–5527
Roose J, Huls G, van Beest M et al (1999) Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285:1923–1926
Staal FJ, Clevers HC (2005) WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol 5:21–30
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480
Vermeulen L, De Sousa EMF, van der Heijden M et al (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 12:468–476
Wielenga VJ, Smits R, Korinek V et al (1999) Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 154:515–523
Strutt DI, Weber U, Mlodzik M (1997) The role of RhoA in tissue polarity and Frizzled signalling. Nature 387:292–295
Winter CG, Wang B, Ballew A et al (2001) Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105:81–91
Ridley AJ, Hall A (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399
Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483–1487
Weeraratna AT, Jiang Y, Hostetter G et al (2002) Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1:279–288
Ouko L, Ziegler TR, Gu LH, Eisenberg LM, Yang VW (2004) Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem 279:26707–26715
Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT (2000) The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 16:279–283
Wang HY, Malbon CC (2003) Wnt signaling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science 300:1529–1530
Derksen PW, Tjin E, Meijer HP et al (2004) Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA 101:6122–6127
Sukhdeo K, Mani M, Zhang Y et al (2007) Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci USA 104:7516–7521
Kramps T, Peter O, Brunner E et al (2002) Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109:47–60
Mani M, Carrasco DE, Zhang Y et al (2009) BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res 69:7577–7586
Dutta-Simmons J, Zhang Y, Gorgun G et al (2009) Aurora kinase A is a target of Wnt/beta-catenin involved in multiple myeloma disease progression. Blood 114:2699–2708
Reya T, Duncan AW, Ailles L et al (2003) A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423:409–414
van de Wetering M, de Lau W, Clevers H (2002) WNT signaling and lymphocyte development. Cell 109(Suppl):S13–S19
Ashihara E, Kawata E, Nakagawa Y et al (2009) beta-catenin small interfering RNA successfully suppressed progression of multiple myeloma in a mouse model. Clin Cancer Res 15:2731–2738
Lepourcelet M, Chen YN, France DS et al (2004) Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 5:91–102
Chim CS, Pang R, Fung TK, Choi CL, Liang R (2007) Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia 21:2527–2536
Qiang YW, Shaughnessy JD Jr, Yaccoby S (2008) Wnt3a signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood 112:374–382
Edwards CM, Edwards JR, Lwin ST et al (2008) Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood 111:2833–2842
Gunn WG, Krause U, Lee N, Gregory CA (2011) Pharmaceutical inhibition of glycogen-synthetase-kinase-3-beta reduces multiple myeloma-induced bone disease in a novel murine plasmacytoma xenograft model. Blood 117(5):1641–1651
Piazza F, Manni S, Tubi LQ et al (2010) Glycogen Synthase Kinase-3 regulates multiple myeloma cell growth and bortezomib-induced cell death. BMC Cancer 10:526
Noda T, Nagano H, Takemasa I et al (2009) Activation of Wnt/beta-catenin signalling pathway induces chemoresistance to interferon-alpha/5-fluorouracil combination therapy for hepatocellular carcinoma. Br J Cancer 100:1647–1658
Cimbora-Zovko T, Ambriovic-Ristov A, Loncarek J, Osmak M (2007) Altered cell-cell adhesion in cisplatin-resistant human carcinoma cells: a link between beta-catenin/plakoglobin ratio and cisplatin resistance. Eur J Pharmacol 558:27–36
Flahaut M, Meier R, Coulon A et al (2009) The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta-catenin pathway. Oncogene 28:2245–2256
De Toni F, Racaud-Sultan C, Chicanne G et al (2006) A crosstalk between the Wnt and the adhesion-dependent signaling pathways governs the chemosensitivity of acute myeloid leukemia. Oncogene 25:3113–3122
Kobune M, Chiba H, Kato J et al (2007) Wnt3/RhoA/ROCK signaling pathway is involved in adhesion-mediated drug resistance of multiple myeloma in an autocrine mechanism. Mol Cancer Ther 6:1774–1784
Bjorklund CC, Ma W, Wang ZQ et al (2011) Evidence of a role for activation of Wnt/{beta}-catenin signaling in the resistance of plasma cells to lenalidomide. J Biol Chem 286(13):11009–11020
Qiang YW, Walsh K, Yao L et al (2005) Wnts induce migration and invasion of myeloma plasma cells. Blood 106:1786–1793
Roodman GD (2004) Pathogenesis of myeloma bone disease. Blood Cells Mol Dis 32:290–292
Sezer O (2005) Myeloma bone disease. Hematology 10(Suppl 1):19–24
Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935
Pearse RN, Sordillo EM, Yaccoby S et al (2001) Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA 98:11581–11586
Vanderkerken K, De Leenheer E, Shipman C et al (2003) Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res 63:287–289
Abe M, Hiura K, Wilde J et al (2004) Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 104:2484–2491
Yaccoby S, Wezeman MJ, Henderson A et al (2004) Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res 64:2016–2023
Yaccoby S, Wezeman MJ, Zangari M et al (2006) Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica 91:192–199
Li X, Pennisi A, Yaccoby S (2008) Role of decorin in the antimyeloma effects of osteoblasts. Blood 112:159–168
Mukherjee S, Raje N, Schoonmaker JA et al (2008) Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest 118:491–504
Giuliani N, Rizzoli V, Roodman GD (2006) Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood 108:3992–3996
Feng R, Anderson G, Xiao G et al (2007) SDX-308, a nonsteroidal anti-inflammatory agent, inhibits NF-kappaB activity, resulting in strong inhibition of osteoclast formation/activity and multiple myeloma cell growth. Blood 109:2130–2138
Anderson G, Gries M, Kurihara N et al (2006) Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood 107:3098–3105
Stewart JP, Shaughnessy JD Jr (2006) Role of osteoblast suppression in multiple myeloma. J Cell Biochem 98:1–13
Baron R, Rawadi G, Roman-Roman S (2006) Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol 76:103–127
Gong Y, Slee RB, Fukai N et al (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523
Kato M, Patel MS, Levasseur R et al (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314
Kulkarni NH, Onyia JE, Zeng Q et al (2006) Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res 21:910–920
Qiang YW, Chen Y, Brown N et al (2010) Characterization of Wnt/beta-catenin signalling in osteoclasts in multiple myeloma. Br J Haematol 148:726–738
Boyden LM, Mao J, Belsky J et al (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521
Glass DA II, Bialek P, Ahn JD et al (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764
Li J, Sarosi I, Cattley RC et al (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39:754–766
Morvan F, Boulukos K, Clement-Lacroix P et al (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934–945
Qiang YW, Hu B, Chen Y et al (2009) Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood 113:4319–4330
Tian E, Zhan F, Walker R et al (2003) The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349:2483–2494
Politou MC, Heath DJ, Rahemtulla A et al (2006) Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer 119:1728–1731
Kaiser M, Mieth M, Liebisch P et al (2008) Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol 80:490–494
Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD Jr (2008) Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone 42:669–680
Hurson CJ, Butler JS, Keating DT et al (2007) Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Musculoskelet Disord 8:12
Colla S, Zhan F, Xiong W et al (2007) The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood 109:4470–4477
Shaughnessy JD Jr, Barlogie B (2003) Interpreting the molecular biology and clinical behavior of multiple myeloma in the context of global gene expression profiling. Immunol Rev 194:140–163
Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr (2007) Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood 109:2106–2111
Oshima T, Abe M, Asano J et al (2005) Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood 106:3160–3165
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Fulciniti, M., Carrasco, D.R. (2013). Role of Wnt Signaling Pathways in Multiple Myeloma Pathogenesis. In: Munshi, N., Anderson, K. (eds) Advances in Biology and Therapy of Multiple Myeloma. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4666-8_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4666-8_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4665-1
Online ISBN: 978-1-4614-4666-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)