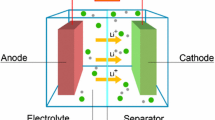
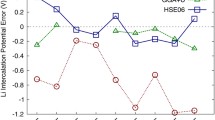
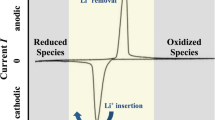
Abstract
In the field of energy materials, the computational modeling of electrochemical devices such as fuel cells, rechargeable batteries, photovoltaic cells, or photo-batteries that combine energy conversion and storage represent a great challenge for theoreticians. Given the wide variety of issues related to the modeling of each of these devices, this chapter is restricted to the study of rechargeable batteries (accumulators) and, more particularly, Li-ion batteries. The aim of this chapter is to emphasize some of the key problems related to the theoretical and computational treatment of these complex systems and to present some of the state-of-the-art computational techniques and methodologies being developed in this area to meet one of the greatest challenges of our century in terms of energy storage.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
The acceptance criterium is such that (i) if \(\Delta E \le 0\), the new structure is used as the new guess structure or (ii) if \(\Delta E > 0\) the new structure is assigned to a probability \(P(E) = {\text{e}}^{{ - (\Delta E/k_{B} T)}}\), thus leading to a canonical ensemble of atomic configurations at T.
- 2.
We should note here that not only the Li+ ions but also the constituting elements of the host matrix can show some disorder in the structure.
- 3.
In surface calculations, boundary conditions are applied to a unit cell consisting in a material slab characterized by its (hkl) Miller indices and a vacuum layer. The spatial reference for the potential is set by the position in the vacuum layer where the electrostatic potential is a local extremum. Surfaces are generally built in such a way that the two surfaces of the active slab do not interact and are symmetrically related. This way, the middle of the interslab distance corresponds to the reference potential position.
- 4.
The interslab distance must account for the slab extension and is therefore calculated as the difference in the z-coordinate of the atoms lying on either sides of the layer minus their atomic radii.
References
Plett GL (2004)Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs Part 2. Modeling and identification. J Power Sources 134(2):262–276
Doyle M, Newman J (1995) Modeling the performance of rechargeable lithium-based cells: design correlations for limiting cases. J Power Sources 54(1):46–51
Darling R, Newman J (1998) Modeling Side Reactions in Composite LiyMn2O4 Electrodes. J Electrochem Soc 145(3):990–998
Ramadass P, Haran B, Gomadam PM, White R, Popov BN (2004) Development of first principles capacity fade model for Li-Ion cells. J Electrochem Soc 151(2):A196–A203
Safari M, Morcrette M, Teyssot A, Delacourt C (2009) Multimodal physics-based aging model for life prediction of Li-Ion batteries. J Electrochem Soc 156(3):A145–A153
Safari M, Delacourt C (2011) Mathematical Modeling of Lithium Iron Phosphate Electrode: Galvanostatic Charge/Discharge and Path Dependence. J Electrochem Soc 158(2):A63–A174
Young WM, Elcock EW (1966) Monte Carlo studies of vacancy migration in binary ordered alloys: IProc Phys Soc 89:735–746
Fowler R, Guggenheim EA (1939) Statistical thermodynamics. Cambridge University Press, Cambridge, p 7
Lebon G, Jou D, Casas-Vázquez J (2008) Understanding non-equilibrium thermodynamics: foundations, applications, frontiers. Springer, Berlin. ISBN: 978-3-540-74252-4
Karma A, Rappel W-J (1998) Quantitative phase-field modeling of dendritic growth in two and three dimensions. Phys Rev E 57(4):4323–4349
Franco AA, Schott P, Jallut C, Maschke B (2007) A multi-scale dynamic mechanistic model for the transient analysis of PEFCs. Fuel Cells 7(2):99–117
Bazant M (2013) Theory of chemical kinetics and charge transfer based on nonequilibrium thermodynamics. Acc Chem Res 46(5):1144–1160
Franco AA (2013) Multiscale modelling and numerical simulation of rechargeable lithium ion batteries: concepts, methods and challenges. RSC Adv 3:13027–13058
Padhi AK, Nanjundaswamy KS, Masquelier C, Okada S, Goodenough JB (1997) Effect of structure on the Fe3+/Fe2+ Redox Couple in Iron Phosphates. J Electrochem Soc 144:1609–1613
Saubanère M, Ben Yahia M, Lebègue S, Doublet M-L (2014) An intuitive and efficient method for cell voltage prediction of lithium and sodium-ion batteries. Nat Commun 5:5559
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136(3):B864–871
Kohn W, Sham LJ (1965) Self-Consistent equations including exchange and correlation Effects. Phys Rev 140(4):A1133–A1138
von Barth U, Hedin L (1972) A local exchange-correlation potential for the spin polarized case. J Phys C: Solid State Phys 5(13):1629–1642
Wang Y, Perdew JP (1991) Correlation hole of the spin-polarized electron gas, with exact small-wave-vector and high-density scaling. Phys Rev B 44(24):13298–13307
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45(23):13244–13249
Becke AD (1993) Density functional thermochemistry III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Perdew JP, Ernzerhof M, Burke K (1996) Rationale for mixing exact exchange with density functional approximations. J Chem Phys 105(22):9982–9985
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: The PBE0 model. J Chem Phys 110(13):6158–6170
Heyd J, Scuseria GE, Ernzerhof M (2003) Hybrid functionals based on a screened coulomb potential. J Chem Phys 118(18):8207–8215
Anisimov VI, Zaanen J, Andersen OK (1991) Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys Rev B 44(3):943–954
Liechtenstein AI, Anisimov VI, Zaanen J (1995) Density-functional theory and strong interactions: orbital ordering in Mott-Hubbard insulator. Phys Rev B 52(8):R5467–R5470
Dudarev SL, Botton GA, Savrasov SY, Humphreys CJ, Sutton AP (1998) Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys Rev B 57(3):1505–1509
Kulik HJ, Cococcioni M, Scherlis DA, Marzari N (2006) Density functional theory in transition-metal chemistry: a self-consistent Hubbard U approach. Phys Rev Lett 97(10):103001-1-4
Aydinol MK, Kohan AF, Ceder G, Cho K, Joannopoulos J (1997) Ab initio study of lithium intercalation in metal oxides and metal dichalcogenides. Phys Rev B 56(3):1354–1365
Feynman RP (1939) Forces in Molecules. Phys Rev 56(4):340–343
Oganov AR, Glass CW (2006) Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J Chem Phys 124(24):244704–1–15
Ceder G, Morgan D, Fischer C, Tibbetts K, Curtarolo S (2006) Data-Mining-Driven quantum mechanics for the prediction of structure. MRS Bull 31:981–985
See the Material Project website: https://www.materialsproject.org/
Kirkpatrick S, Gelatt CD, Vecchi MP (1983) Optimization by simulated annealing. Science 220(4598):671–680
Wales D, Doye J (1997) Global Optimization by basin-hopping and the lowest energy structures of lennard-jones clusters containing up to 110 Atoms. J Phys Chem A 101(28):5111–5118
Grotendorst J (ed) (2000) Modern methods and algorithms of quantum chemistry. John von Neumann Institute for Computing, Jülich, NIC Series, Vol 1, pp 301–449. ISBN 3-00-005618-1
Gonze X, Lee C (1997) Dynamical matrices, Born effective charges, dielectric permittivity tensors, and interatomic force constants from density-functional perturbation theory. Phys Rev B 55(16):10355–10368
Ben Yahia M, Lemoigno F, Beuvier T, Filhol JS, Richard-Plouet M, Brohan L, Doublet ML (2009) Updated references for the structural, electronic, and vibrational properties of TiO2(B) bulk using first-principles density functional theory calculations. J Chem Phys 130(20):204501-1-11
Kikuchi R (1951) A Theory of Cooperative Phenomena. Phys Rev 81(6):988–1003
Sanchez JM, Ducastelle F, Gratias D (1984) Generalized cluster description of multicomponent systems Physica 128:334–350
Persson K, Hinuma Y, Meng YS, Van der Ven A, Ceder G (2010) Thermodynamic and kinetic properties of the Li-graphite system from first-principles calculations. Phys Rev B 82(12):125416-1-9
Van der Ven A, Aydinol MK, Ceder G, Kresse G, Hafner J (1998) First-principles investigation of phase stability in LixCoO2. Phys Rev B 58(6):2975–2987
Van der Ven A, Aydinol MK, Ceder G (1998) First Principles evidence for stage ordering in LixCoO2. J Electrochem Soc 145(6):2149–2155
Van der Ven A, Ceder G, Asta M, Tepesch PD (2001) First-principles theory of ionic diffusion with nondilute carriers. Phys Rev B 64(18):184307-1-17
Mueller T, Ceder G (2009) Bayesian approach to cluster expansions. Phys Rev B 80(2):024103-1-13
Bethe HA (1935) Statistical theory of superlattices. Proc Roy Soc London A 150:552–575
Filhol J-S, Combelles C, Yazami R, Doublet M-L (2008) Phase diagrams for systems with low free energy variation: A coupled theory/experiments method applied to Li-graphite. J Phys Chem C 112(10):3982–3988
Cabana J, Monconduit L, Larcher D, Palacin MR (2010) Beyond Intercalation-Based Li-Ion Batteries: The State of the art and challenges of electrode materials reacting through conversion reactions. Adv Mater 22:E170–E192
Wang L, Maxisch T, Ceder G (2006) Oxidation energies of transition metal oxides within the GGA+U framework. Phys Rev B 73(19):195107-1-6
Chevrier VL, Ong SP, Armiento R, Chan MKY, Ceder G (2010) Hybrid density functional calculations of redox potentials and formation energies of transition metal compounds. Phys Rev B 82(7):075122-1-11
Zhou F, Cococcioni M, Marianetti CA, Morgan D, Ceder G (2004) First-principles prediction of redox potentials in transition-metal compounds with LDA+U. Phys Rev B 70(23):235121–1–8
Hautier G, Jain A, Ong SP, Kang B, Moore C, Doe R, Ceder G (2011) Phosphates as Lithium-Ion Battery Cathodes: An evaluation based on high-throughput ab Initio calculations. Chem Mater 23:3495–3508
Ben Yahia M, Lemoigno F, Rousse G, Boucher F, Tarascon JM, Doublet ML (2012) Origin of the 3.6 V to 3.9 V voltage increase in the LiFeSO4F cathodes for Li-ion batteries. Energy Environ Sci 5:9584–9594
Frayret C, Villesuzanne A, Spaldin N, Bousquet E, Chotard J-N, Recham N, Tarascon J-M (2010) LiMSO4F (M = Fe, Co and Ni): promising new positive electrode materials through the DFT microscope. Phys Chem Chem Phys 12:15512–15522
Jain A, Hautier G, Ping Ong S, Moore CJ, Fischer CC, Persson KA, Ceder G (2011) Formation enthalpies by mixing GGA and GGA + U calculations. Phys Rev B 84(4):045115-1-10
Hautier G, Fischer C, Ehrlacher V, Jain A, Ceder G (2011) Data Mined ionic substitutions for the discovery of new compounds. Inorg Chem 50(2):656–663
Mueller T, Hautier G, Jain A, Ceder G (2011) Evaluation of tavorite-structured cathode materials for lithium-Ion batteries using high-throughput computing Chem Mater 23(17):3854–3862
Jain A, Hautier G, Moore CJ, Ping Ong S, Fischer CC, Mueller T, Persson KA, Ceder G (2011) A high-throughput infrastructure for density functional theory calculations Comp Mater Sci 50:2295–2310
Ping Ong P, Richards WD, Jain A, Hautier G, Kocher M, Cholia S, Gunter D, Chevrier V, Ceder G (2013) Python Materials Genomics (pymatgen): A robust, open-source python library for materials analysis. Comp Mater Sci 68:314–319
Van der Ven A, Ceder G (2005) Vacancies in ordered and disordered binary alloys treated with the cluster expansion. Phys Rev B 71(5):054102-1-7
Bergerhoff G, Hundt R, Sievers R, Brown ID (1983) The inorganic crystal structure data base. J Chem Inf Comput Sci 23:66–69
Belsky A, Hellenbrandt M, Karen VL, Luksch P (2002) New developments in the inorganic crystal structure database (ICSD): accessibility in support of materials research and design. Acta Crystallogr A 58:364–369
Grazulis S et al (2009) Crystallography open database an open-access collection of crystal structures. J Appl Crystallogr 42:726–729
Downs RT, Hall-Wallace M (2003) The american mineralogist crystal structure database. Am Mineral 88:247–250
Eyring H (1935) The Activated complex in chemical reactions. J Chem Phys 3:107–115
Laidler K, King C (1983) Development of transition-state theory. J Phys Chem 87(15):2657–2664
Henkelman G, Jóhannesson G, Jónsson H (2000) Methods for finding saddle points and minimum energy paths. In: Schwartz SD (ed) Progress on theoretical chemistry and physics. Kluwer Academic Publishers, pp 269–300
Henkelman G, Uberuaga BP, Jónsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys 113(22):9901–9904
Yan X, Gouissem A, Sharma P (2015) Atomistic insights into Li-ion diffusion in amorphous silicon. Mech Mater. doi:10.1016/j.mechmat.2015.04.001
Malik R, Burch D, Bazant M, Ceder G (2010) Particle size dependence of the ionic diffusivity. Nano Lett 10(10):4123–4127
Arora P, White RE, Doyle M (1998) Capacity Fade Mechanisms and Side Reactions in Lithium-Ion Batteries. J Electrochem Soc 145(10):3647–3667
Vetter J, Novák P, Wagner MR, Veit C, Möller K-C, Besenhard JO, Winter M, Wohlfahrt-Mehrens M, Vogler C, Hammouche A (2005) Ageing mechanisms in lithium-ion batteries. J Power Sources 147(1–2):269–281
Christensen J, Newman J (2005) Cyclable lithium and capacity loss in Li-Ion cells. J Electrochem Soc 152(4):A818–830
Schmickler W, Santos E (eds) (2010) Interfacial electrochemistry, 2nd edn. Springer, Berlin
Nørskov JK, Rossmeisl J, Logadottir A, Lindqvist L, Kitchin JR, Bligaard T, Jonsson H (2004) Origin of the overpotential for oxygen reduction at a Fuel-cell cathode. J Phys Chem B 108(46):17886–17892
Lozovoi AY, Alavi A, Kohanoff J, Lynden-Bell RM (2001) Ab initio simulation of charged slabs at constant chemical potential. J Chem Phys 115(4):1661–1669
Filhol J-S, Neurock M (2006) Angew Chem Int Ed Elucidation of the Electrochemical Activation of Water over Pd by First Principles 45(3):402–406
Filhol J-S, Bocquet M-L (2007) Charge control of the water monolayer/Pd interface Chem. Phys Lett 438(4–6):203–207
Taylor CD, Wasileski SA, Filhol J-S, Neurock M (2006) First principles reaction modeling of the electrochemical interface: Consideration and calculation of a tunable surface potential from atomic and electronic structure. Phys Rev B 73(16):165402–1–16
Mamatkoulov M, Filhol J-S (2011) An ab initio study of electrochemical vs. electromechanical properties: the case of CO adsorbed on a Pt (111) surface. Phys Chem Chem Phys 13(17):7675–7684
Filhol J-S, Doublet M-L (2013) An ab initio study of surface electrochemical disproportionation: the case of a water monolayer adsorbed on a Pd (111) surface Catal Today 202:87–97
Lespes N, Filhol J-S (2015) Using the electrochemical dimension to build water/Ru (0001) phase diagram. Surf Sci 631:8–16
Dalverny A-L, Filhol J-S, Doublet M-L (2011) Interface Electrochemistry in Conversion Reactions for Li-Ion Batteries. J Mat Chem 21:10134–10142
Lespes N, Filhol J-S (2015) Using Implicit Solvent in Ab Initio Electrochemical Modeling: Investigating Li+/Li Electrochemistry at a Li/Solvent Interface. J Comp Theo Chem. 11(7):3375–3382 doi:10.1021/acs.jctc.5b00170
Ando K, Hynes JT (1997) Molecular mechanism of Hcl acid ionization in water: Ab initio potential energy surfaces and Monte Carlo simulations. J Phys Chem B 101(49):10464–10478
Del Popolo MG, Lynden-Bell RM, Kohanoff J (2005) Ab initio molecular dynamics simulation of a room temperature ionic liquid. J Phys Chem B 109(12):5895–5902
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105(8):2999–3093
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-Pcm solvation model. J Comput Chem 24(6):669–681
Jinnouchi R, Anderson AB (2008) Electronic structure calculations of liquid-solid interfaces: combination of density functional theory and modified Poisson-Boltzmann theory. Phys Rev B 77(24):245417-1-18
Andreussi O, Dabo I, Marzari N (2012) Revised self-consistent continuum solvation in electronic-structure calculations. J Chem Phys 136(6):064102-1-20
Steinmann S, Michel C, Schwiedernoch R, Filhol J-S, Sautet P (2015) Modeling the HCOOH/CO2 Electrocatalytic Reaction, When Details Are Key. Chem Phys Chem. 16(11):2307–2311. doi:10.1002/cphc.201500187
Filhol J-S, Doublet M-L (2014) Conceptual surface electrochemistry and new redox descriptors. J Phys Chem C 118(33):19023–19031
Khatib R, Dalverny A-L, saubanère M, Gaberscek M, Doublet ML (2013) Origin of the voltage hysteresis in the CoP conversion material for Li-Ion batteries. J Phys Chem C 117:837–849
Meggiolaro D, Gigli G, Paolone A, Reale P, Doublet M-L, Brutti S (2015) Origin of the voltage hysteresis of MgH 2 electrodes in Lithium. J Phys Chem C 119:17044–17052
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag London
About this chapter
Cite this chapter
Saubanère, M., Filhol, JS., Doublet, ML. (2016). Atomistic Modeling of Electrode Materials for Li-Ion Batteries: From Bulk to Interfaces. In: Franco, A., Doublet, M., Bessler, W. (eds) Physical Multiscale Modeling and Numerical Simulation of Electrochemical Devices for Energy Conversion and Storage. Green Energy and Technology. Springer, London. https://doi.org/10.1007/978-1-4471-5677-2_1
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5677-2_1
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5676-5
Online ISBN: 978-1-4471-5677-2
eBook Packages: EnergyEnergy (R0)