Abstract
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. The prevalence of AF is increasing as the median age of the population is on rise. Incidence of AF increases with age, as one in five individuals over age 85 has AF. Several clinical conditions are associated with AF, most importantly ischemic heart disease, diabetes, hypertension, cardiomyopathy, valvular heart disease, and heart failure.
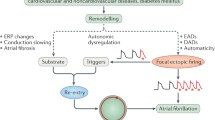
AF is a complex disease and its pathogenesis is multifactorial. The relatively recent discovery of the importance of the pulmonary veins (PV) in the origin of triggers and generation of AF has helped us better understand the pathophysiology of AF. The region of the PVs and the adjoining posterior left atrium (PLA) possesses a unique, heterogeneous pattern of myocyte orientation; the abrupt changes in electrical conduction patterns that result in these regions make the atria more susceptible to AF (by setting up substrate for reentry). Another important factor that contributes to atrial arrhythmogenesis is the unique expression of key ion channels in the atrium. Compared to the ventricles, some ion channels, e.g., I KAch and I Kur, are predominantly expressed in atrial myocytes; these channels, by contributing to shortening of refractoriness, make the atria more vulnerable to fibrillation. In addition, the PVs and PLA have a unique pattern of ion channel and gap junction expression that makes the atria susceptible to AF. Ca2+ dysregulation has also been noted to play an important role in generation and maintenance of AF, with excitation-contraction coupling being significantly altered in fibrillating myocytes (as compared to normal atrial myocytes). In addition to ion channel, gap junction, and Ca2+ remodeling, structural remodeling – specifically fibrosis – has been implicated in AF initiation and maintenance, especially in the setting of structural heart disease, e.g., heart failure. Key signaling pathways that are thought to create fibrosis in the atrium including TGF-β signaling, oxidative stress, and angiotensin II signaling. The autonomic nervous system is also thought to play an important role in generation of AF, with both the sympathetic and parasympathetic thought to play an important role in AF initiation and maintenance. Recently, Genome-wide association studies have also provided us with new insights into genetic predisposition in AF generation.
In this chapter, we have reviewed in detail the role of these pathophysiological mechanisms in the generation and maintenance of AF.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–53.
Hobbs FD, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess. 2005;9:iii–iv, ix–x, 1–74.
Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Seward JB, Bailey KR, et al. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980 to 2000: report of a community-based study. Stroke. 2005;36:2362–6.
Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6.
Stevenson WG, Stevenson LW, Middlekauff HR, et al. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol. 1996;28:1458–63.
Mays DJ, Foose JM, Philipson LH, Tamkun MM. Localization of the Kv1.5 K_ channel protein in explanted cardiac tissue. J Clin Invest. 1995;96:282–92.
Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K_ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–76.
Blaauw Y, Schotten U, van Hunnik A, Neuberger HR, Allessie MA. Cardioversion of persistent atrial fibrillation by a combination of atrial specific and non-specific class III drugs in the goat. Cardiovasc Res. 2007;75:89–98.
De Haan S, Greiser M, Harks E, Blaauw Y, van Hunnik A, Verheule S, et al. AVE0118, blocker of the transient outward current [I(to)] and ultrarapid delayed rectifier current [I(Kur)], fully restores atrial contractility after cardioversion of atrial fibrillation in the goat. Circulation. 2006;114:1234–42.
Dobrzynski H, Marples DD, Musa H, Yamanushi TT, Henderson Z, Takagishi Y, et al. Distribution of the muscarinic K_ channel proteins Kir3.1 and Kir3 4 in the ventricle, atrium, and sinoatrial node of heart. J Histochem Cytochem. 2001;49:1221–34.
Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988;405:123–45.
Golod DA, Kumar R, Joyner RW. Determinants of action potential initiation in isolated rabbit atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 1998;274:H1902–13.
Haïssaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66.
Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–5.
Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74.
Ehrlich JR, Cha TJ, Zhang L, Chartier D, Melnyk P, Hohnloser SH, Nattel S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol. 2003;551:801–13.
Melnyk P, Ehrlich JR, Pourrier M, Villeneuve L, Cha TJ, Nattel S. Comparison of ion channel distribution and expression in cardiomyocytes of canine pulmonary veins versus left atrium. Cardiovasc Res. 2005;65:104–16.
Maupoil V, Bronquard C, Freslon JL, Cosnay P, Findlay I. Ectopic activity in the rat pulmonary vein can arise from simultaneous activation of alpha1- and beta1-adrenoceptors. Br J Pharmacol. 2007;150:899–905.
Henning B, Wit AL. The time course of action potential repolarization affects delayed afterdepolarization amplitude in atrial fibers of the canine coronary sinus. Circ Res. 1984;55:110–5.
Tseng GN, Wit AL. Effects of reducing [Na_]o on catecholamine-induced delayed afterdepolarizations in atrial cells. Am J Physiol Heart Circ Physiol. 1987;253:H115–25.
Wit AL, Cranefield PF. Triggered and automatic activity in the canine coronary sinus. Circ Res. 1977;41:434–45.
Nguyen BL, Fishbein MC, Chen LS, Chen PS, Masroor S. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm. 2006;6:454–60.
Verheule S, Wilson E, Arora R, Engle SK, Scott L, Olgin J. Tissue structure and connexin expression of canine pulmonary veins. Cardiovasc Res. 2002;55:727–38.
Kholova I, Kautzner J. Morphology of atrial myocardial extensions into human caval veins: a postmortem study in patients with and without atrial fibrillation. Circulation. 2004;110:483–8.
Hassink RJ, Aretz HT, Ruskin J, Keane D. Morphology of atrial myocardium in human pulmonary veins: a postmortem analysis in patients with and without atrial fibrillation. J Am Coll Cardiol. 2003;42:1108–14.
Hocini M, Ho SY, Kawara T, Linnenbank AC, Potse M, Shah D, et al. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation. 2002;105:2442–8.
Chou CC, Nihei M, Zhou S, Tan A, Kawase A, Macias ES, et al. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–97.
Arora R, Verheule S, Scott L, Navarrete A, Katari V, Wilson E, et al. Arrhythmogenic substrate of the pulmonary veins assessed by high-resolution optical mapping. Circulation. 2003;107:1816–21.
Kumagai K, Ogawa M, Noguchi H, Yasuda T, Nakashima H, Saku K. Electrophysiologic properties of pulmonary veins assessed using a multielectrode basket catheter. J Am Coll Cardiol. 2004;43:2281–9.
Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124(20):2264–74.
Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325.
Wijffels M, Kirchhof C, Dorland R, Allessie M. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68.
Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. Circ Res. 1973;33:54–62.
Comtois P, Kneller J, Nattel S. Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace. 2005;7 Suppl 2:10–20.
Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70.
Dobrev D, Voigt N, Wehrens XH. The ryanodine receptor channel as a molecular motif in atrial fibrillation: pathophysiological and therapeutic implications. Cardiovasc Res. 2011;89(4):734–43.
MacLennan DH, Chen SR. Store overload-induced Ca2+ release as a triggering mechanism for CPVT and MH episodes caused by mutations in RYR and CASQ genes. J Physiol. 2009;587(pt 13):3113–5.
Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415(6868):219–26.
Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–14.
Tsai CTLL, Kuo KT, Hwang JJ, Hsieh CS, Hsu KL, Tseng CD, et al. Angiotensin II activates signal transducer and activators of transcription 3 via Rac1 in atrial myocytes and fibroblasts: implication for the therapeutic effect of statin in atrial structural remodeling. Circulation. 2008;117:344–55.
He X, Gao X, Peng L, Wang S, Zhu Y, Ma H, et al. Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific arkadia-mediated downregulation of Smad7. Circ Res. 2011;108(2):164–75.
Gassanov N, Brandt MC, Michels G, Lindner M, Er F, Hoppe UC. Angiotensin II–induced changes of calcium sparks and ionic currents in human atrial myocytes: potential role for early remodeling in atrial fibrillation. Cell Calcium. 2006;39(2):175–86.
Verheule S, Sato T, Everett 4th T, Engle SK, Otten D, Rubart-von der Lohe M, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta 1. Circ Res. 2004;94(11):1458–65.
Everett 4th T, Olgin JE. Atrial fibrosis and mechanism of atrial fibrillation. Heart Rhythm. 2007;4(3 Suppl):S24–7.
Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118(1):10–24.
Nakajima H, Nakajima HO, Salchar O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86(5):571–9.
Lamirault G, Gaborit N, Le Meur N, Chevalier C, Lande G, Demolombe S, et al. Gene expression profile associated with chronic atrial fibrillation and underlying valvular heart disease in man. J Mol Cell Cardiol. 2006;40(1):173–84.
Lee KW, Everett 4th TH, Rahmutula D, Guerra JM, Wilson E, Ding C, Olgin JE. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114(16):1703–12.
Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–9.
Ponten A, Folestad EB, Pietras K, Eriksson U. Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res. 2005;97(10):1036–45.
Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol. 2011;50(3):408–16.
Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104(2):174–80.
Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89(6):E32–8.
Kim YH, Lim DS, Lee JH, Shim WJ, Ro YM, Park GH, et al. Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp Mol Med. 2003;35(5):336–49.
Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley Jr SC. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53(9):1652–7.
Ramlawi B, Otu H, Mieno S, Boodhwani M, Sodha NR, Clements RT, et al. Oxidative stress and atrial fibrillation after cardiac surgery: a case–control study. Ann Thorac Surg. 2007;84(4):1166–72.
Korantzopoulos P, Kolettis TM, Kountouris E, Dimitroula V, Karanikis P, Pappa E, et al. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol. 2005;102(2):321–6.
Marin FRV, Climent V, Garcia A, Marco P, Lip GYH. Is thrombogenesis in atrial fibrillation related to matrix metalloproteinase-1 and its inhibitor, TIMP-1? Stroke. 2003;34:1181–6.
Goette ARC, Nepple K, Lendeckel U. Proteases and arrhythmias. In: Lendeckel U, editor. Proteases in tissue remodelling of lung and heart. New York: Plenum; 2003. p. 191–390.
Hunt MJ, Aru GM, Hayden MR, Moore CK, Hoit BD, Tyagi SC. Induction of oxidative stress and disintegrin metalloproteinase in human heart end-stage failure. Am J Physiol Lung Cell Mol Physiol. 2002;283:L239–45.
Crabtree GROE. NFAT signalling: choreographing the social lives of cells. Cell Mol Life Sci. 2002;109(Suppl):S67–79.
Brundel BJ, Ausma J, van Gelder IC, Van der Want JJ, van Gilst WH, Crijns HJ, Henning RH. Activation of proteolysis by calpains and structural changes in human paroxysmal and persistent atrial fibrillation. Cardiovasc Res. 2002;54:380–9.
Bukowska A, Hirte D, Wolke C, Striggow F, Röhnert P, Huth C, Klein HU, Goette A. Activation of the calcineurin signaling pathway induces atrial hypertrophy during atrial fibrillation. Cell Mol Life Sci. 2006;63:333–42.
Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106(6):1134–44.
Shiroshita-Takeshita A, Mitamura H, Ogawa S, Nattel S. Rate-dependence of atrial tachycardia effects on atrial refractoriness and atrial fibrillation maintenance. Cardiovasc Res. 2009;81(1):90–7.
Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–25.
Attuel P, Childers R, Cauchemez B, Poveda J, Mugica J, Coumel P. Failure in the rate adaptation of the atrial refractory period: its relationship to vulnerability. Int J Cardiol. 1982;2:179–97.
Boutjdir M, Le Heuzey JY, Lavergne T, Chauvaud S, Guize L, Carpentier A, Peronneau P. Inhomogeneity of cellular refractoriness in human atrium: factor of arrhythmia? Pacing Clin Electrophysiol. 1986;9:1095–100.
Misier AR, Opthof T, van Hemel NM, Defauw JJ, de Bakker JM, Janse MJ, van Capelle FJ. Increased dispersion of “refractoriness” in patients with idiopathic paroxysmal atrial fibrillation. J Am Coll Cardiol. 1992;19:1531–5.
Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–31.
Brundel BJ, Van Gelder IC, Henning RH, Tieleman R, Tuinenburg AE, Wietses M, et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–90.
Skasa M, Jungling E, Picht E, Schondube F, Luckhoff A. L-type calcium currents in atrial myocytes from patients with persistent and non-persistent atrial fibrillation. Basic Res Cardiol. 2001;96:151–9.
Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, et al. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K_ channels. J Am Coll Cardiol. 2001;37:926–32.
Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999;84(7):776–84.
Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, et al. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res. 2008;103(8):845–54.
Goette A, Arndt M, Rocken C, Staack T, Bechtloff R, Reinhold D, et al. Calpains and cytokines in fibrillating human atria. Am J Physiol Heart Circ Physiol. 2002;283:H264–72.
Grammer JB, Bosch RF, Kuhlkamp V, Seipel L. Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation. J Cardiovasc Electrophysiol. 2000;11:626–33.
Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K_ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–81.
Dobrev D, Wettwer E, Kortner A, Knaut M, Schuler S, Ravens U. Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovasc Res. 2002;54:397–404.
Zhang H, Garratt CJ, Zhu J, Holden AV. Role of up-regulation of IK1 in action potential shortening associated with atrial fibrillation in humans. Cardiovasc Res. 2005;66:493–502.
Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, et al. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–81.
El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114(7):670–80.
Christ T, Boknik P, Wöhrl S, Wettwer E, Graf EM, Bosch RF, et al. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110(17):2651–7.
Luo X, et al. Critical role of microRNAs miR-26 and miR-101 in atrial electrical remodeling in experimental atrial fibrillation. Circulation. 2010;122, A19435.
Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, et al. The G protein-gated potassium current I(K, ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112(24):3697–706.
Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L, Nattel S. Kir3-based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113(14):1730–7.
Karle CA, Zitron E, Zhang W, Wendt-Nordahl G, Kathöfer S, Thomas D, et al. Human cardiac inwardly-rectifying K+ channel Kir(2.1b) is inhibited by direct protein kinase C-dependent regulation in human isolated cardiomyocytes and in an expression system. Circulation. 2002;106(12):1493–9.
Voigt N, Friedrich A, Bock M, Wettwer E, Christ T, Knaut M, et al. Differential phosphorylation dependent regulation of constitutively active and muscarinic receptor-activated IK, ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–37.
Workman AJ, Kane KA, Rankin AC. Characterisation of the Na, K pump current in atrial cells from patients with and without chronic atrial fibrillation. Cardiovasc Res. 2003;59:593–602.
Brandt MC, Priebe L, Bohle T, Sudkamp M, Beuckelmann DJ. The ultrarapid and the transient outward K_ current in human atrial fibrillation. Their possible role in postoperative atrial fibrillation. J Mol Cell Cardiol. 2000;32:1885–96.
Schotten U, Duytschaever M, Ausma J, Eijsbouts S, Neuberger HR, Allessie M. Electrical and contractile remodeling during the first days of atrial fibrillation go hand in hand. Circulation. 2003;107:1433–9.
Duffy HS, Wit AL. Is there a role for remodeled connexins in AF? No simple answers. J Mol Cell Cardiol. 2008;44:4–13.
Wilhelm M, Kirste W, Kuly S, Amann K, Neuhuber W, Weyand M, et al. Atrial distribution of connexin 40 and 43 in patients with intermittent, persistent, and postoperative atrial fibrillation. Heart Lung Circ. 2006;15:30–7.
Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, et al. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol. 2001;38:883–91.
Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–79.
Takeuchi S, Akita T, Takagishi Y, Watanabe E, Sasano C, Honjo H, Kodama I. Disorganization of gap junction distribution in dilated atria of patients with chronic atrial fibrillation. Circ J. 2006;70:575–82.
Nattel S. Defining “culprit mechanisms” in arrhythmogenic cardiac remodeling. Circ Res. 2004;94:1403–5.
Goette A, Juenemann G, Peters B, Klein H, Roessner A, Huth C, Rocken C. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. 2002;54:390–6.
Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999;100:376–80.
Vermes ETJ, Bourassa MG, Racine N, Levesque S, White M, Guerra PG, Ducharme A. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003;107:2926–31.
Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95.
Boyden PA, Hoffman BF. The effects on atrial electrophysiology and structure of surgically induced right atrial enlargement in dogs. Circ Res. 1981;49:1319–31.
Neuberger HR, Schotten U, Verheule S, Eijsbouts S, Blaauw Y, van Hunnik A, Allessie M. Development of a substrate of atrial fibrillation during chronic atrioventricular block in the goat. Circulation. 2005;111:30–7.
Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–63.
Spach MS, Heidlage JF, Barr RC, Dolber PC. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm. 2004;1:500–15.
Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4:S61–4.
Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ, Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res. 2002;90:E73–87.
Liang BT, Frame LH, Molinoff PB. Beta 2-adrenergic receptors contribute to catecholamine-stimulated shortening of action potential duration in dog atrial muscle. Proc Natl Acad Sci. 1985;82:4521–5.
Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47(6):1196–206.
Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2(6):624–31.
Patterson E, Yu X, Huang S, Garrett M, Kem DC. Suppression of autonomic-mediated triggered firing in pulmonary vein preparations, 24 hours postcoronary artery ligation in dogs. J Cardiovasc Electrophysiol. 2006;17(7):763–70.
Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43(3):483–90.
Crawford T, Chugh A, Good E, et al. Clinical value of noninducibility by high-dose isoproterenol versus rapid atrial pacing after catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:13–20.
Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–34.
Kalifa J, Jalife J, Zaitsev AV, et al. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–71.
Arruda M, Natale A. Ablation of permanent AF: adjunctive strategies to pulmonary veins isolation: targeting AF NEST in sinus rhythm and CFAE in AF. J Interv Card Electrophysiol. 2008;23:51–7.
Porter M, Spear W, Akar JG, et al. Prospective study of atrial fibrillation termination during ablation guided by automated detection of fractionated electrograms. J Cardiovasc Electrophysiol. 2008;19:613–20.
Aimé-Sempé C, Folliguet T, Rücker-Martin C, et al. Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol. 1999;34:1577–86.
Katritsis D, Giazitzoglou E, Sougiannis D, et al. Complex fractionated atrial electrograms at anatomic sites of ganglionated plexi in atrial fibrillation. Europace. 2009;11:308–15.
Arora R, Ulphani JS, Villuendas R, Ng J, Harvey L, Thordson S, et al. Neural substrate for atrial fibrillation: implications for targeted parasympathetic blockade in the posterior left atrium. Am J Physiol Heart Circ Physiol. 2008;294:H134–44.
Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48(1):132–43.
Arora R, Ng J, Ulphani J, Mylonas I, Subacius H, Shade G, et al. Unique autonomic profile of the pulmonary veins and posterior left atrium. J Am Coll Cardiol. 2007;49:1340–8.
Ng J, Villuendas R, Cokic I, Schliamser JE, Gordon D, Koduri H, et al. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–96.
Ehrlich JR, Zicha S, Coutu P, Hebert TE, Nattel S. Atrial fibrillation-associated minK38G/S polymorphism modulates delayed rectifier current and membrane localization. Cardiovasc Res. 2005;67(3):520–8.
Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42(3):240–4.
Ozgen N, Dun W, Sosunov EA, Anyukhovsky EP, Hirose M, Duffy HS, et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res. 2007;75(4):758–69.
Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, et al. Ablation of a Ca2+ − activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587(pt 5):1087–100.
Nyberg MT, Stoevring B, Behr ER, Ravn LS, McKenna WJ, Christiansen M. The variation of the sarcolipin gene (SLN) in atrial fibrillation, long QT syndrome and sudden arrhythmic death syndrome. Clin Chim Acta. 2007;375(1–2):87–91.
Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421(6923):634–9.
Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15(14):2185–91.
Li Q, Huang H, Liu G, Lam K, Rutberg J, Green MS, et al. Gain-of-function mutation of Nav1.5 in atrial fibrillation enhances cellular excitability and lowers the threshold for action potential firing. Biochem Biophys Res Commun. 2009;380(1):132–7.
Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299(5604):251–4.
Hong K, Bjerregaard P, Gussak I, Brugada R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16(4):394–6.
Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, et al. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332(4):1012–9.
Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115(4):442–9.
Eckardt L, Kirchhof P, Loh P, Schulze-Bahr E, Johna R, Wichter T, et al. Brugada syndrome and supraventricular tachyarrhythmias: a novel association? J Cardiovasc Electrophysiol. 2001;12:680–5.
Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, et al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116:1569–76.
Lee CH, Liu PY, Lin LJ, Chen JH, Tsai LM. Clinical characteristics and outcomes of hypertrophic cardiomyopathy in Taiwan: a tertiary center experience. Clin Cardiol. 2007;30:177–82.
Losi MA, Betocchi S, Aversa M, Lombardi R, Miranda M, et al. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2004;94:895–900.
Spitzer SG, Richter P, Knaut M, Schuler S. Treatment of atrial fibrillation in open heart surgery: the potential role of microwave energy. Thorac Cardiovasc Surg. 1999;47 Suppl 3:374–8.
White CM, Giri S, Tsikouris JP, Dunn A, Felton K, Reddy P, Kluger J. A comparison of two individual amiodarone regimens to placebo in open heart surgery patients. Ann Thorac Surg. 2002;74:69–74.
Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–11.
Auer J, Weber T, Berent R, Ng CK, Lamm G, Eber B. Risk factors of postoperative atrial fibrillation after cardiac surgery. J Card Surg. 2005;20:425–31.
Tsikouris JP, Kluger J, Song J, White CM. Changes in P-wave dispersion and P-wave duration after open heart surgery are associated with the peak incidence of atrial fibrillation. Heart Lung. 2001;30:466–71.
Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–8.
Baker WL, White CM, Kluger J, Denowitz A, Konecny CP, Coleman CI. Effect of perioperative corticosteroid use on the incidence of postcardiothoracic surgery atrial fibrillation and length of stay. Heart Rhythm. 2007;4:461–8.
Halonen J, Halonen P, Jarvinen O, Taskinen P, Auvinen T, Tarkka M, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA. 2007;297:1562–7.
Kalman JM, Munawar M, Howes LG, Louis WJ, Buxton BF, Gutteridge G, Tonkin AM. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60:1709–15.
White HD, Antman EM, Glynn MA, Collins JJ, Cohn LH, Shemin RJ, Friedman PL. Efficacy and safety of timolol for prevention of supraventricular tachyarrhythmias after coronary artery bypass surgery. Circulation. 1984;70:479–84.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Arora, R., Koduri, H.K. (2014). Mechanisms of Atrial Fibrillation. In: Kibos, A., Knight, B., Essebag, V., Fishberger, S., Slevin, M., Țintoiu, I. (eds) Cardiac Arrhythmias. Springer, London. https://doi.org/10.1007/978-1-4471-5316-0_31
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5316-0_31
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5315-3
Online ISBN: 978-1-4471-5316-0
eBook Packages: MedicineMedicine (R0)