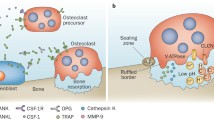
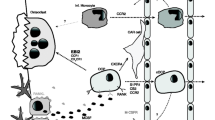
Abstract
Macrophages are a key player to regulate rheumatoid arthritis pathogenesis from onset to remission. They can alter innate functions under microenvironmental conditions. To understand heterogeneous functions of macrophages in rheumatoid arthritis, several activated statuses of macrophages should be mimicked in vitro. Here, we describe basic protocols for macrophage polarization and osteoclast differentiation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013) Tissue-resident macrophages. Nat Immunol 14(10):986–995. https://doi.org/10.1038/ni.2705
Epelman S, Lavine KJ, Randolph GJ (2014) Origin and functions of tissue macrophages. Immunity 41(1):21–35. https://doi.org/10.1016/j.immuni.2014.06.013
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Investig 122(3):787–795. https://doi.org/10.1172/jci59643
Orecchioni M, Ghosheh Y, Pramod AB, Ley K (2019) Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS–) vs. alternatively activated macrophages. Front Immunol:10. https://doi.org/10.3389/fimmu.2019.01084
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3(1):23–35. https://doi.org/10.1038/nri978
Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T, Castegna A (2019) The metabolic signature of macrophage responses. Front Immunol 10:1462. https://doi.org/10.3389/fimmu.2019.01462
Obach M, Navarro-Sabaté À, Caro J, Kong X, Duran J, Gómez M et al (2004) 6-phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem 279(51):53562–53570. https://doi.org/10.1074/jbc.m406096200
Li Y, Zhang P, Wang C, Han C, Meng J, Liu X et al (2013) Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species. J Biol Chem 288(23):16225–16234. https://doi.org/10.1074/jbc.m113.454538
Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA et al (2014) Metabolic reprogramming of macrophages. J Biol Chem 289(11):7884–7896. https://doi.org/10.1074/jbc.m113.522037
Abhishek KJ, Stanley CCH, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E et al (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42(3):419–430. https://doi.org/10.1016/j.immuni.2015.02.005
Haschemi A, Kosma P, Gille L, Charles RE, Charles FB, Starkl P et al (2012) The Sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab 15(6):813–826. https://doi.org/10.1016/j.cmet.2012.04.023
Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM et al (2014) Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 15(9):846–855. https://doi.org/10.1038/ni.2956
Tavakoli S, Downs K, Short JD, Nguyen HN, Lai Y, Jerabek PA et al (2017) Characterization of macrophage polarization states using combined measurement of 2-deoxyglucose and glutamine accumulation. Arterioscler Thromb Vasc Biol 37(10):1840–1848. https://doi.org/10.1161/atvbaha.117.308848
Smith D, The M (2011) Normal synovium. Open Rheumatol J 5(1):100–106. https://doi.org/10.2174/1874312901105010100
Kurowska-Stolarska M, Alivernini S (2017) Synovial tissue macrophages: friend or foe? RMD Open 3(2):e000527. https://doi.org/10.1136/rmdopen-2017-000527
Culemann S, Gruneboom A, Nicolas-Avila JA, Weidner D, Lammle KF, Rothe T et al (2019) Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 572(7771):670–675. https://doi.org/10.1038/s41586-019-1471-1
Alivernini S, Macdonald L, Elmesmari A, Finlay S, Tolusso B, Gigante MR et al (2020) Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat Med 26(8):1295–1306. https://doi.org/10.1038/s41591-020-0939-8
Hasegawa T, Kikuta J, Sudo T, Matsuura Y, Matsui T, Simmons S et al (2019) Identification of a novel arthritis-associated osteoclast precursor macrophage regulated by FoxM1. Nat Immunol 20(12):1631–1643. https://doi.org/10.1038/s41590-019-0526-7
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Saeki, N., Nakata, A. (2024). Macrophage Polarization and Osteoclast Differentiation. In: Liu, S. (eds) Rheumatoid Arthritis. Methods in Molecular Biology, vol 2766. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3682-4_26
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3682-4_26
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3681-7
Online ISBN: 978-1-0716-3682-4
eBook Packages: Springer Protocols