Abstract
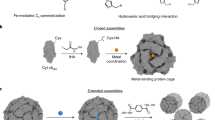
Natural protein assemblies have encouraged scientists to create large supramolecular systems consisting of various protein motifs. In the case of hemoproteins containing heme as a cofactor, several approaches have been reported to form artificial assemblies with various structures such as fibers, sheets, networks, and cages. This chapter describes the design, preparation, and characterization of cage-like micellar assemblies for chemically modified hemoproteins including hydrophilic protein units attached to hydrophobic molecules. Detailed procedures are described for constructing specific systems using cytochrome b562 and hexameric tyrosine-coordinated heme protein as hemoprotein units with heme-azobenzene conjugate and poly-N-isopropylacrylamide as attached molecules.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Luo Q, Hou C, Bai Y, Wang R, Liu J (2016) Protein assembly: versatile approaches to construct highly ordered nanostructures. Chem Rev 116:13571–13632
Kobayashi N, Arai R (2017) Design and construction of self-assembling supramolecular protein complexes using artificial and fusion proteins as nanoscale building blocks. Curr Opin Biotech 46:57–65
van Dun S, Ottmann C, Milroy LG, Brunsveld L (2017) Supramolecular chemistry targeting proteins. J Am Chem Soc 139:13960–13968
Fegan A, White B, Carlson JCT, Wagner CR (2010) Chemically controlled protein assembly: techniques and applications. Chem Rev 110:3315–3336
Lai YT, Cascio D, Yeates TO (2012) Structure of a 16-nm cage designed by using protein oligomers. Science 336:1129
Hsia Y, Bale JB, Gonen S, Shi D, Sheffler W, Fong KK, Nattermann U, Xu C, Huang P, Ravichandran R, Yi S, Davis TN, Gonen T, King NP, Baker D (2016) Design of a hyperstable 60-subunit protein icosahedron. Nature 535:136–139
Burazerovic S, Gradinaru J, Pierron J, Ward TR (2007) Hierarchical self-assembly of one-dimensional streptavidin bundles as a collagen mimetic for the biomineralization of calcite. Angew Chem Int Ed 46:5510–5514
Nguyen TK, Negishi H, Abe S, Ueno T (2019) Construction of supramolecular nanotubes from protein crystals. Chem Sci 10:1046–1051
Malay AD, Miyazaki N, Biela A, Chakraborti S, Majsterkiewicz K, Stupka I, Kaplan CS, Kowalczyk A, Piette BMAG, Hochberg GKA, Wu D, Wrobel TP, Fineberg A, Kushwah MS, Kelemen M, Vavpetič P, Pelicon P, Kukura P, Benesch JLP, Iwasaki K, Heddle JG (2019) An ultra-stable gold-coordinated protein cage displaying reversible assembly. Nature 569:438–442
Simon AJ, Zhou Y, Ramasubramani V, Glaser J, Pothukuchy A, Gollihar J, Gerberich JC, Leggere JC, Morrow BR, Jung C, Glotzer SC, Taylor DW, Ellington AD (2019) Supercharging enables organized assembly of synthetic biomolecules. Nat Chem 11:204–212
Oohora K, Hayashi T (2014) Hemoprotein-based supramolecular assembling systems. Curr Opin Chem Biol 19:154–161
Hirota S (2019) Oligomerization of cytochrome c, myoglobin, and related heme proteins by 3D domain swapping. J Inorg Biochem 194:170–179
Hirota S, Hattori Y, Nagao S, Taketa M, Komori H, Kamikubo H, Wang Z, Takahashi I, Negi S, Sugiura Y, Kataoka M, Higuchi Y (2010) Cytochrome c polymerization by successive domain swapping at the C-terminal helix. Proc Natl Acad Sci 107:12854–12859
Zhang M, Nakanishi T, Yamanaka M, Nagao S, Yanagisawa S, Shomura Y, Shibata N, Ogura T, Higuchi Y, Hirota S (2017) Rational design of domain-swapping-based c-type cytochrome heterodimers by using chimeric proteins. Chembiochem 18:1712–1715
Hayashi Y, Yamanaka M, Nagao S, Komori H, Higuchi Y, Hirota S (2016) Domain swapping oligomerization of thermostable c-type cytochrome in E. coli cells. Sci Rep 6:19334
Miyamoto T, Kuribayashi M, Nagao S, Shomura Y, Higuchi Y, Hirota S (2015) Domain-swapped cytochrome cb562 dimer and its nanocage encapsulating a Zn-SO4 cluster in the internal cavity. Chem Sci 6:7336–7342
Churchfield LA, Tezcan FA (2019) Design and construction of functional supramolecular metalloprotein assemblies. Acc Chem Res 52:345–355
Radford RJ, Tezcan FA (2009) A superprotein triangle driven by nickel(II) coordination: exploiting non-natural metal ligands in protein self-assembly. J Am Chem Soc 131:9136–9137
Ni TW, Tezcan FA (2010) Structural characterization of a microperoxidase inside a metal-directed protein cage. Angew Chem Int Ed 49:7014–7018
Song WJ, Tezcan FA (2014) A designed supramolecular protein assembly with in vivo enzymatic activity. Science 346:1525–1528
Brodin JD, Smith SJ, Carr JR, Tezcan FA (2015) Designed, helical protein nanotubes with variable diameters from a single building block. J Am Chem Soc 137:10468–10471
Golub E, Subramanian RH, Esselborn J, Alberstein RG, Bailey JB, Chiong JA, Yan X, Booth T, Baker TS, Tezcan FA (2020) Constructing protein polyhedra via orthogonal chemical interactions. Nature 578:172–176
Oohora K, Onoda A, Hayashi T (2012) Supramolecular assembling systems formed by heme–heme pocket interactions in hemoproteins. Chem Commun 48:11714–11726
Kitagishi H, Oohora K, Yamaguchi H, Sato H, Matsuo T, Harada A, Hayashi T (2007) Supramolecular hemoprotein linear assembly by successive interprotein heme−heme pocket interactions. J Am Chem Soc 129:10326–10327
Oohora K, Fujimaki N, Kajihara R, Watanabe H, Uchihashi T, Hayashi T (2018) Supramolecular hemoprotein assembly with a periodic structure showing heme–heme exciton coupling. J Am Chem Soc 140:10145–10148
Oohora K, Kajihara R, Jiromaru M, Kitagishi H, Hayashi T (2019) Arginine residues provide a multivalent effect for cellular uptake of a hemoprotein assembly. Chem Lett 48:295–298
Soon JW, Oohora K, Hirayama S, Hayashi T (2021) A supramolecular assembly of hemoproteins formed in a star-shaped structure via heme–heme pocket interactions. Int J Mol Sci 22:1012
Kitagishi H, Kakikura Y, Yamaguchi H, Oohora K, Harada A, Hayashi T (2009) Self-assembly of one- and two-dimensional hemoprotein systems by polymerization through heme–heme pocket interactions. Angew Chem Int Ed 48:1271–1274
Oohora K, Onoda A, Kitagishi H, Yamaguchi H, Harada A, Hayashi T (2011) A chemically-controlled supramolecular proteinpolymer formed by a myoglobin-based self-assembly system. Chem Sci 2:1033–1038
Oohora K, Onuma Y, Tanaka Y, Onoda A, Hayashi T (2017) A supramolecular assembly based on an engineered hemoprotein exhibiting a thermal stimulus-driven conversion to a new distinct supramolecular structure. Chem Commun 53:6879–6882
Jeoung JH, Pippig DA, Martins BM, Wagener N, Dobbek H (2007) HTHP: a novel class of hexameric, tyrosine-coordinated heme proteins. J Mol Biol 368:1122–1131
Oohora K, Hirayama S, Mashima T, Hayashi T (2020) Supramolecular dimerization of a hexameric hemoprotein via multiple pyrene-pyrene interactions. J Porphyrins Phthalocyanines 24:259–267
Oohora K, Hirayama S, Uchihashi T, Hayashi T (2020) Construction of a hexameric hemoprotein sheet and direct observation of dynamic process of its formation. Chem Lett 49:186–190
Hirayama S, Oohora K, Uchihashi T, Hayashi T (2020) Thermoresponsive micellar assembly constructed from a hexameric hemoprotein modified with poly(N-isopropylacrylamide) toward an artificial light-harvesting system. J Am Chem Soc 142:1822–1831
Wan X, Liu S (2010) Fabrication of a thermoresponsive biohybrid double hydrophilic block copolymer by a cofactor reconstitution approach. Macromol Rapid Commun 31:2070–2076
Reynhout IC, Cornelissen JJLM, Nolte RJM (2007) Self-assembled architectures from biohybrid triblock copolymers. J Am Chem Soc 129:2327–2332
Acknowledgments
We acknowledge supports from JSPS KAKENHI JP15H05804, JP15K21707, JP20H00403, and JP20H02755.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Oohora, K., Hayashi, T. (2023). Preparation of Cage-Like Micellar Assemblies of Engineered Hemoproteins. In: Ueno, T., Lim, S., Xia, K. (eds) Protein Cages. Methods in Molecular Biology, vol 2671. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3222-2_5
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3222-2_5
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3221-5
Online ISBN: 978-1-0716-3222-2
eBook Packages: Springer Protocols