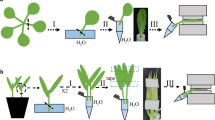
Abstract
Different parts of a plant can be simultaneously exposed to very different conditions, for example a leaf moving in and out of shadow. In addition to local responses, transmission of information between different tissues and organs is thought to affect the coordination of overall responses to changing environmental conditions. An important adaptive role is played by the stomata, which regulate the evaporation of water vapor and supply of CO2 for photosynthesis. Here, we describe a method to study the effect of distally triggered systemic signals on stomatal conductance. The experimental set up, consisting of a growth chamber and a leaf gas exchange measuring system, enables time-resolved measurements on an intact leaf while maintaining a full control over the environmental conditions of the measured leaf and the whole seedling. The method can be used as a powerful tool to study short- and long-term stomatal responses to changes in different environmental variables, such as light.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Merilo E, Laanemets K, Hu H et al (2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol 162:1652–1668. https://doi.org/10.1104/pp.113.220608
Hashimoto M, Negi J, Young J et al (2006) Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol 8:391–397. https://doi.org/10.1038/ncb1387
Hõrak H, Sierla M, Tõldsepp K et al (2016) A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure. Plant Cell 28:2493–2509. https://doi.org/10.1105/tpc.16.00131
Karpinski S, Reynolds H, Karpinska B et al (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284:654–657. https://doi.org/10.1126/science.284.5414.654
Suzuki N, Miller G, Salazar C et al (2013) Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25:3553–3569. https://doi.org/10.1105/tpc.113.114595
Miller G, Schlauch K, Tam R et al (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2:ra45. https://doi.org/10.1126/scisignal.2000448
Choi W-G, Toyota M, Kim S-H et al (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci 111:6497–6502. https://doi.org/10.1073/pnas.1319955111
Szechyńska-Hebda M, Kruk J, Górecka M et al (2010) Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22:2201–2218. https://doi.org/10.1105/tpc.109.069302
Koziolek C, Grams TEE, Schreiber U et al (2004) Transient knockout of photosynthesis mediated by electrical signals. New Phytol 161:715–722. https://doi.org/10.1111/j.1469-8137.2004.00985.x
Hlaváčková V, Krchňák P, Nauš J et al (2006) Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta 225:235–244. https://doi.org/10.1007/s00425-006-0325-x
Kaiser H, Grams TEE (2006) Rapid hydropassive opening and subsequent active stomatal closure follow heat-induced electrical signals in Mimosa pudica. J Exp Bot 57:2087–2092. https://doi.org/10.1093/jxb/erj165
Grams TEE, Lautner S, Felle HH et al (2009) Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ 32:319–326. https://doi.org/10.1111/j.1365-3040.2008.01922.x
Gallé A, Lautner S, Flexas J et al (2013) Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ 36:542–552. https://doi.org/10.1111/j.1365-3040.2012.02594.x
Devireddy AR, Zandalinas SI, Gómez-Cadenas A et al (2018) Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci Signal 11:eaam9514. https://doi.org/10.1126/scisignal.aam9514
Devireddy AR, Arbogast J, Mittler R (2020) Coordinated and rapid whole-plant systemic stomatal responses. New Phytol 225:21–25. https://doi.org/10.1111/nph.16143
Ehonen S, Hölttä T, Kangasjärvi J (2020) Systemic signaling in the regulation of stomatal conductance. Plant Physiol 182:1829–1832. https://doi.org/10.1104/pp.19.01543
Zandalinas SI, Cohen IH, Fritschi FB, Mittler R (2020) Coordinated systemic stomatal responses in soybean. Plant Physiol 183:1428–1431. https://doi.org/10.1104/pp.20.00511
Hõrak H, Fountain L, Dunn JA et al (2020) Dynamic thermal imaging confirms local but not fast systemic ABA responses. Plant Cell Environ 44:885–899. https://doi.org/10.1111/pce.13973
Farmer E, Farmer E, Mousavi S, Lenglet A (2013) Leaf numbering for experiments on long distance signalling in Arabidopsis. Protoc Exch. https://doi.org/10.1038/protex.2013.071
Vahisalu T, Puzõrjova I, Brosché M et al (2010) Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J 62:442–453. https://doi.org/10.1111/j.1365-313X.2010.04159.x
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210x.2009.00001.x
Wood SN (2006) Generalized additive models: an introduction with R, 2nd edn. Chapman & Hall/CRC, Boca Raton, FL
Rose NL, Yang H, Turner SD, Simpson GL (2012) An assessment of the mechanisms for the transfer of lead and mercury from atmospherically contaminated organic soils to lake sediments with particular reference to Scotland, UK. Geochim Cosmochim Acta 82:113–135. https://doi.org/10.1016/J.GCA.2010.12.026
Acknowledgments
We thank Mikael Brosché and Pedro Aphalo for fruitful discussions and critical comments on the manuscript and Tuomas Puukko for providing the pictures. The authors were supported by the Academy of Finland (grants 333703 and 336359 to M.S.), Ella and Georg Ehrnrooth Foundation (M.S.), Finnish Cultural Foundation (M.S.), and Alfred Kordelin Foundation (S.E.).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Ehonen, S., Sierla, M. (2022). Gas Exchange Measurements in Systemic Signaling Studies. In: Yoshida, T. (eds) Abscisic Acid. Methods in Molecular Biology, vol 2462. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2156-1_9
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2156-1_9
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2155-4
Online ISBN: 978-1-0716-2156-1
eBook Packages: Springer Protocols