Abstract
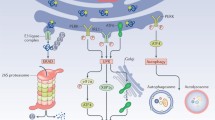
Hepatocyte lipotoxicity is a hallmark of nonalcoholic steatohepatitis (NASH), and lipid induced liver injury occurs, in part, via activation of endoplasmic reticulum (ER) stress. Consequently, the unfolded protein response (UPR) is initiated, driven by three key ER transmembrane proteins, resulting in downstream responses that are dynamic and interconnected. Thus, careful interrogation of these pathways is required to investigate the complex role of ER stress in NASH. Herein, we describe different mechanisms of, and in vitro assays for assessment of lipotoxic ER stress in mouse hepatocytes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Malhi H, Kaufman RJ (2011) Endoplasmic reticulum stress in liver disease. J Hepatol 54:795–809
Song MJ, Malhi H (2019) The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacol Ther 203:107401
Hetz C, Zhang K, Kaufman RJ (2020) Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 21:421–438
Kakazu E, Mauer AS, Yin M et al (2016) Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res 57:233–245
Speziali G, Liesinger L, Gindlhuber J et al (2018) Myristic acid induces proteomic and secretomic changes associated with steatosis, cytoskeleton remodeling, endoplasmic reticulum stress, protein turnover and exosome release in HepG2 cells. J Proteome 181:118–130
Pina F, Yagisawa F, Obara K et al (2018) Sphingolipids activate the endoplasmic reticulum stress surveillance pathway. J Cell Biol 217:495–505
Martinez L, Torres S, Baulies A et al (2015) Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget 6:41479–41496
Malhi H, Bronk SF, Werneburg NW et al (2006) Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281:12093–12101
Lee AH, Scapa EF, Cohen DE et al (2008) Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320:1492–1496
Volmer R, van der Ploeg K, Ron D (2013) Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A 110:4628–4633
Puri P, Mirshahi F, Cheung O et al (2008) Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134:568–576
Averous J, Bruhat A, Jousse C et al (2004) Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem 279:5288–5297
Pfaffenbach KT, Gentile CL, Nivala AM et al (2010) Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab 298:E1027–E1035
Wang Y, Shen J, Arenzana N et al (2000) Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem 275:27013–27020
Yamamoto K, Takahara K, Oyadomari S et al (2010) Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell 21:2975–2986
Rutkowski DT, Wu J, Back SH et al (2008) UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 15:829–840
Beriault DR, Werstuck GH (2013) Detection and quantification of endoplasmic reticulum stress in living cells using the fluorescent compound, Thioflavin T. Biochim Biophys Acta 1833:2293–2301
Tam AB, Roberts LS, Chandra V et al (2018) The UPR Activator ATF6 Responds to Proteotoxic and Lipotoxic Stress by Distinct Mechanisms. Dev Cell 46:327–343.e327
Fun XH, Thibault G (2020) Lipid bilayer stress and proteotoxic stress-induced unfolded protein response deploy divergent transcriptional and non-transcriptional programmes. Biochim Biophys Acta Mol Cell Biol Lipids 1865:158449
de Almeida IT, Cortez-Pinto H, Fidalgo G et al (2002) Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr 21:219–223
Oliveira AF, Cunha DA, Ladriere L et al (2015) In vitro use of free fatty acids bound to albumin: a comparison of protocols. BioTechniques 58:228–233
Spector AA, John K, Fletcher JE (1969) Binding of long-chain fatty acids to bovine serum albumin. J Lipid Res 10:56–67
Kakisaka K, Cazanave SC, Fingas CD et al (2012) Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol 302:G77–G84
Richieri GV, Kleinfeld AM (1995) Unbound free fatty acid levels in human serum. J Lipid Res 36:229–240
Klaunig JE, Goldblatt PJ, Hinton DE et al (1981) Mouse liver cell culture. I. Hepatocyte isolation. In Vitro 17:913–925
Marciniak SJ, Yun CY, Oyadomari S et al (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077
Haataja L, Gurlo T, Huang CJ et al (2008) Many commercially available antibodies for detection of CHOP expression as a marker of endoplasmic reticulum stress fail specificity evaluation. Cell Biochem Biophys 51:105–107
Higa A, Taouji S, Lhomond S et al (2014) Endoplasmic reticulum stress-activated transcription factor ATF6alpha requires the disulfide isomerase PDIA5 to modulate chemoresistance. Mol Cell Biol 34:1839–1849
Kracht MJL, de Koning EJP, Hoeben RC et al (2018) Bioluminescent reporter assay for monitoring ER stress in human beta cells. Sci Rep 8:17738
Rong J, Pass I, Diaz PW et al (2015) Cell-based high-throughput luciferase reporter gene assays for identifying and profiling chemical modulators of endoplasmic reticulum signaling protein, IRE1. J Biomol Screen 20:1232–1245
Xi Y, Garshott DM, Brownell AL et al (2015) Cantharidins induce ER stress and a terminal unfolded protein response in OSCC. J Dent Res 94:320–329
Nagy Z, Comer S, Smolenski A (2018) Analysis of protein phosphorylation using Phos-Tag gels. Curr Protoc Protein Sci 93:e64
Acknowledgments
Grant support: This work is supported by NIH grants DK111378 (H.M.) and the Mayo Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Parthasarathy, G., Malhi, H. (2022). Assessment of Lipotoxic Endoplasmic Reticulum (ER) Stress in Nonalcoholic Steatohepatitis (NASH). In: Sarkar, D. (eds) Non-Alcoholic Steatohepatitis. Methods in Molecular Biology, vol 2455. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2128-8_19
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2128-8_19
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2127-1
Online ISBN: 978-1-0716-2128-8
eBook Packages: Springer Protocols