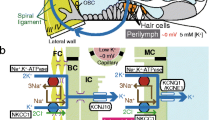
Abstract
Functionally and anatomically, the laboratory mouse inner ear is comparable to other mammals, except that it is considerably smaller and operates over a higher acoustic frequency range. Other than miniaturization of methods that might be applied to the inner ears of guinea pigs, gerbils, or chinchillas, the major difference lies in fewer points of access, due both to small number of cochlear turns \( \left(2\raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$4$}\right.\right) \) and reduced access to cochlear scalae in any single turn. These features do not particularly complicate auditory brainstem, compound action potential, or distortion product emission recording. Due to the close proximity to generators, these responses can be quite large in mice. Instead, they present challenges for endocochlear potential (EP) measurement and for manipulations and measurements of inner ear fluids. Without appropriate modifications, considerable technical challenges and potential pitfalls can render such measurements uninterpretable in mice. This chapter outlines experimental techniques for targeting inner ear fluid manipulations and fluid measures in mice. We specifically consider methods for EP measurement, perilymph sampling, and introduction of chemical agents into the middle or inner ear.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- CA:
-
Cyanoacrylate adhesive
- EP:
-
Endocochlear potential
- IHCs:
-
Inner hair cells
- IT:
-
Intratympanic
- OHCs:
-
Outer hair cells
- PSCC:
-
Posterior semicircular canal
- RWM:
-
Round window membrane
- TM:
-
Tympanic membrane
References
Bowl MR, Dawson SJ (2015) The mouse as a model for age-related hearing loss-a mini-review. Gerontology 61:149–157
Ohlemiller KK, Jones SM, Johnson KR (2016) Application of mouse models to research in hearing and balance. J Assoc Res Otolaryngol 17:1–31
Hosoya M, Fujioka M, Kobayashi R et al (2016) Overlapping expression of anion exchangers in the cochlea of a non-human primate suggests functional compensation. Neurosci Res 110:1–10
Hosoya M, Fujioka M, Ogawa K et al (2016) Distinct expression patterns of causative genes responsible for hereditary progressive hearing loss in non-human primate Cochlea. Sci Rep 6:1–12
Crowson MG, Hertzano R, Tucci D (2017) Emerging therapies for sensorineural hearing loss. Otol Neurotol 38:792–803
Wang J, Puel JL (2018) Toward cochlear therapies. Physiol Rev 98:2477–2522
Bielefeld EC, Kobel MJ (2019) Advances and challenges in pharmaceutical therapies to prevent and repair cochlear injuries from noise. Front Cell Neurosci 13:285
Szeto B, Chiang H, Valentini C, Yu M, Kysar JW, Lalwani AK (2020) Inner ear delivery: challenges and opportunities. Laryngoscope Invest Otolaryngol 5:122–131
Dallos P, Zheng J, Cheatham MA (2006) Prestin and the cochlear amplifier. J Physiol 576:37–42
Carney LH (2012) Peripheral anatomy and physiology. In: Tremblay KL, Burkard RF (eds) Translational perspectives in auditory neuroscience: normal aspects of hearing. Plural, San Diego, pp 91–111
Jahnke K (1975) The fine structure of freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol 336:1–40
Wangemann P (2006) Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576(1):11–21
Gill S, Salt AN (1997) Quantitative differences in endolymphatic calcium and endocochlear potential between pigmented and albino guinea pigs. Hearing Res 113:191–197
Suzuki J, Hashimoto K, Xiao R et al (2017) Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci Rep 7:1–12
Schmiedt RA (2010) Chapter 2: the physiology of cochlear presbycusis. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR (eds) Springer handbook of auditory research, The aging auditory system, vol 34. Springer, New York, pp 9–38
Mercier PP, Lysaght AC, Bandyopadhyay S et al (2012) Energy extraction from the biologic battery in the inner ear. Nat Biotechnol 30:1240–1243. https://doi.org/10.1038/nbt.2394
Tl K (1979) Some observations on negative endocochlear potential during anoxia. Acta Otolaryngol 87:506–516
Hirose K, Liberman MC (2003) Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol 4:339–352
Saunders JC, Garfinkle TJ (1983) Peripheral anatomy and physiology I. In: Willott JF (ed) The auditory psychobiology of the mouse. Charles C. Thomas, Springfield, IL, pp 131–168
Johnstone BM, Johnstone JR, Pugsley ID (1966) Membrane resistance in endolymphatic walls of the first turn of the guinea-pig cochlea. J Acoust Soc Am 40:1398–1404
Schrott A, Melichar I, Popelar J et al (1990) Deterioration of hearing function in mice with neural crest defect. Hearing Res 46:1–7
Liu H, Zhao L (2015) Recording potentials from scala media, saccule and utricle in mice. J Otol 10:87–91
Li Y, Liu H, Zhao X et al (2020) Endolymphatic potential measured from developing and adult mouse inner ear. Front Cell Neurosci 14:431
Ohlemiller KK, Dahl AR, Gagnon PM (2010) Divergent aging characteristics in CBA/J and CBA/CaJ mouse cochleae. J Assoc Res Otolaryngol 11:605–623
Ohlemiller KK, Rosen AD, Gagnon PM (2010) A major effect QTL on chromosome 18 for noise injury to the mouse cochlear lateral wall. Hearing Res 260:47–53
Sewell W (1984) The effects of furosemide on the endocochlear potential and auditory nerve fiber tuning curves in cats. Hearing Res 14:305–314
Steel KP, Barkway C (1989) Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development 107:453–463
Ingham NJ, Carlisle F, Pearson S et al (2016) S1PR2 variants associated with auditory function in humans and endocochlear potential decline in mouse. Sci Rep 6:1–13
Nishio A, Ito T, Cheng H, Fitzgerald TS, Wangemann P, Griffith AJ (2016) Slc26a4 expression prevents fluctuation of hearing in a mouse model of large vestibular aqueduct syndrome. Neuroscience 329:74–82
Ohlemiller KK (2009) Mechanisms and genes in human strial presbycusis from animal models. Brain Res 1277:70–83
Ohlemiller KK, Rosen AR, Rellinger EA et al (2011) Different cellular and genetic basis of noise-related endocochlear potential reduction in CBA/J and BALB/cJ mice. J Assoc Res Otolaryngol 12:45–58
Ohlemiller KK, Kaur T, Warchol ME et al (2018) The endocochlear potential as an indicator of reticular lamina integrity after noise exposure in mice. Hearing Res 361:138–151
Salt AN, Brown DJ, Hartsock JJ et al (2009) Displacements of the organ of Corti by gel injections into the cochlear apex. Hearing Res 250:63–75
Ohlemiller KK, Kiener AL, Gagnon PM (2016) QTL mapping of endocochlear potential differences between C57BL/6J and BALB/cJ mice. J Assoc Res Otolaryngol 17:173–194
Salt AN, Hartsock J, Plontke S et al (2011) Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol Neurotol 16:323–335
Saunders JC, Crumling MA (2001) The outer and middle ear. In: Willott JF (ed) Handbook of mouse auditory research. CRC Press, New York, pp 99–115
Richter CA, Amin S, Linden J et al (2010) Defects in middle ear cavitation cause conductive hearing loss in the Tcof1 mutant mouse. Hum Mol Genet 19:1551–1560
Salt AN, Plontke SK (2009) Principles of local drug delivery to the inner ear. Audiol Neurotol 14:350–360
Salt AN, Hirose K (2018) Communication pathways to and from the inner ear and their contributions to drug delivery. Hearing Res 362:25–37
Schreiner L (1999) Translation: recent experimental and clinical findings retarding an interlabyrinthine connection. Laryngo-Rhino-Otologie 78:387–393
Stöver T, Yagi M, Raphael Y (2000) Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther 7:377–383
Mason MJ (2013) Of mice, moles and guinea pigs: functional morphology of the middle ear in living mammals. Hearing Res 301:4–18
Plontke SK, Hartsock JJ, Gill RM et al (2016) Intracochlear drug injections through the round window membrane: measures to improve drug retention. Audiol Neurotol 21:72–79
György B, Sage C, Indzhykulian AA et al (2017) Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Mol Ther 25:379–391. https://doi.org/10.1016/j.ymthe.2016.12.010
Yoshimura H, Shibata SB, Ranum PT et al (2018) Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci Rep 14:2980. https://doi.org/10.1038/s41598-018-21233-z
Hirose K, Hartsock JJ, Johnson S et al (2014) Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. J Assoc Res Otolaryngol 15:707–719
Acknowledgement
Thanks to Ruth Gill for assistance with some of the figures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Ohlemiller, K.K., Hartsock, J.J., Salt, A.N. (2022). Endocochlear Potential Measures, Local Drug Application, and Perilymph Sampling in the Mouse Inner Ear. In: Groves, A.K. (eds) Developmental, Physiological, and Functional Neurobiology of the Inner Ear. Neuromethods, vol 176. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2022-9_12
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2022-9_12
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2021-2
Online ISBN: 978-1-0716-2022-9
eBook Packages: Springer Protocols