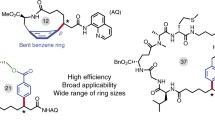
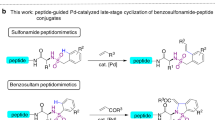
Abstract
Cyclic peptides are an important class of bioactive compounds for the chemical biology and pharmaceutical industry. Chemical synthesis of highly constrained cyclic peptides is often challenging. Here we describe the synthetic strategy of peptide macrocyclization through late-stage palladium-catalyzed C-H activation. These methods utilize endogenous backbone amides in the peptide sequence as directing groups and are efficient in the preparation of small-to-middle size peptide macrocycles.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Gracia SR, Gaus K, Sewald N (2009) Synthesis of chemically modified bioactive peptides: recent advances, challenges and developments for medicinal chemistry. Future Med Chem 1:1289–1310
Henninot A, Collins JC, Nuss JM (2018) The current state of peptide drug discovery: back to the future? J Med Chem 61:1382–1414
Mendive-Tapia L, Preciado S, Garcia J, Ramon R, Kielland N, Albericio F, Lavilla R (2015) New peptide architectures through C-H activation stapling between tryptophan-phenylalanine/tyrosine residues. Nat Commun 6:7160
Driggers EM, Hale SP, Lee J, Terrett NK (2008) The exploration of macrocycles for drug discovery--an underexploited structural class. Nat Rev Drug Discov 7:608–624
Bird GH, Madani N, Perry AF, Princiotto AM, Supko JG, He X, Gavathiotis E, Sodroski JG, Walensky LD (2010) Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc Natl Acad Sci U S A 107:14093–14098
Jewell DA, Swietnicki W, Dunn BM, Malcolm BA (1992) Hepatitis A virus 3C proteinase substrate specificity. Biochemistry 31:7862–7869
Davies JS (2003) The cyclization of peptides and depsipeptides. J Pept Sci 9:471–501
Nguyen GK, Hemu X, Quek JP, Tam JP (2016) Butelase-mediated macrocyclization of d-amino-acid-containing peptides. Angew Chem Int Ed Engl 55:12802–12806
Jo H, Meinhardt N, Wu Y, Kulkarni S, Hu X, Low KE, Davies PL, DeGrado WF, Greenbaum DC (2012) Development of alpha-helical calpain probes by mimicking a natural protein-protein interaction. J Am Chem Soc 134:17704–17713
Gongora-Benitez M, Tulla-Puche J, Albericio F (2014) Multifaceted roles of disulfide bonds. Peptides as therapeutics. Chem Rev 114:901–926
Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ (2004) Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305:1466–1470
Blackwell HE, Sadowsky JD, Howard RJ, Sampson JN, Chao JA, Steinmetz WE, O'Leary DJ, Grubbs RH (2001) Ring-closing metathesis of olefinic peptides: design, synthesis, and structural characterization of macrocyclic helical peptides. J Org Chem 66:5291–5302
White CJ, Yudin AK (2011) Contemporary strategies for peptide macrocyclization. Nat Chem 3:509–524
Reguera L, Rivera DG (2019) Multicomponent reaction toolbox for peptide macrocyclization and stapling. Chem Rev 119:9836–9860
Amore A, van Heerbeek R, Zeep N, van Esch J, Reek JN, Hiemstra H, van Maarseveen JH (2006) Carbosilane dendrimeric carbodiimides: site isolation as a lactamization tool. J Org Chem 71:1851–1860
Gong W, Zhang G, Liu T, Giri R, Yu JQ (2014) Site-selective C(sp3)-H functionalization of di-, tri-, and tetrapeptides at the N-terminus. J Am Chem Soc 136:16940–16946
Liu T, Qiao JX, Poss MA, Yu JQ (2017) Palladium(II)-catalyzed site-selective C(sp(3) )-H alkynylation of oligopeptides: a linchpin approach for oligopeptide-drug conjugation. Angew Chem Int Ed Engl 56:10924–10927
Wang W, Lorion MM, Martinazzoli O, Ackermann L (2018) BODIPY peptide labeling by late-stage C(sp(3) )-H activation. Angew Chem Int Ed Engl 57:10554–10558
Bai Z, Cai C, Sheng W, Ren Y, Wang H (2020) Late-stage peptide macrocyclization via palladium-catalyzed site-selective C-H olefination of tryptophan. Angew Chem Int Ed Engl 59(34):14686–14692
Tan J, Wu J, Liu S, Yao H, Wang H (2019) Macrocyclization of peptidoarylacetamides with self-assembly properties through late-stage palladium-catalyzed C(sp(2))-H olefination. Sci Adv 5:eaaw0323
Bai Z, Wang H (2019) Backbone-enabled peptide macrocyclization through late-stage palladium-catalyzed C–H activation. Synlett. https://doi.org/10.1055/s-0039-1691495
Bai Q, Tang J, Wang H (2019) Functionalization of sulfonamide-containing peptides through late-stage palladium-catalyzed C(sp(3))-H arylation. Org Lett 21:5858–5861
Bai Q, Bai Z, Wang H (2019) Macrocyclization of biaryl-bridged peptides through late-stage palladium-catalyzed C(sp(2))-H arylation. Org Lett 21:8225–8228
Tang J, Chen H, He Y, Sheng W, Bai Q, Wang H (2018) Peptide-guided functionalization and macrocyclization of bioactive peptidosulfonamides by Pd(II)-catalyzed late-stage C-H activation. Nat Commun 9:3383
Tang J, He YD, Chen HF, Sheng WJ, Wang H (2017) Synthesis of bioactive and stabilized cyclic peptides by macrocyclization using C(sp(3))-H activation. Chem Sci 8:4565–4570
Bai Z, Cai C, Yu Z, Wang H (2018) Backbone-enabled directional peptide macrocyclization through late-stage palladium-catalyzed delta-C(sp(2))-H olefination. Angew Chem Int Ed 57:13912–13916
Acknowledgments
This work is supported by NSF of China (Grant 21922703 and 91953112), the Natural Science Foundation of Jiangsu Province (Grant BK20190004) and the Fundamental Research Funds for the Central Universities (Grant 14380138 and 14380131).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Cai, C., Liu, S., Wang, H. (2022). Peptide Macrocyclization Through Palladium-Catalyzed Late-Stage C-H Activation. In: Coppock, M.B., Winton, A.J. (eds) Peptide Macrocycles. Methods in Molecular Biology, vol 2371. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1689-5_3
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1689-5_3
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1688-8
Online ISBN: 978-1-0716-1689-5
eBook Packages: Springer Protocols