Abstract
Emergency admissions due to acute metabolic crisis in patients with diabetes remain some of the most common and challenging conditions. DKA (Diabetic Ketoacidosis), HHS (Hyperglycaemic Hyperosmolar State) and recently focused EDKA (Euglycaemic Diabetic Ketoacidosis) are life-threatening different entities. DKA and HHS have distinctly different pathophysiology but basic management protocols are the same. EDKA is just like DKA but without hyperglycaemia. T1D, particularly children are vulnerable to DKA and T2D, particularly elderly with comorbidities are vulnerable to HHS. But these are not always the rule, these acute conditions are often occur in different age groups with diabetes. It is essential to have a coordinated care from the multidisciplinary team to ensure the timely delivery of right treatment. DKA and HHS, in many instances can present as a mixed entity as well. Mortality rate is higher for HHS than DKA but incidences of DKA are much higher than HHS. The prevalence of HHS in children and young adults are increasing due to exponential growth of obesity and increasing T2D cases in this age group. Following introduction of SGLT2i (Sodium-GLucose co-Transporter-2 inhibitor) for T2D and off-label use in T1D, some incidences of EDKA has been reported. Healthcare professionals should be more vigilant during acute illness in diabetes patients on SGLT2i without hyperglycaemia to rule out EDKA. Middle aged, mildly obese and antibody negative patients who apparently resemble as T2D without any precipitating causes sometime end up with DKA which is classified as KPD (Ketosis-prone diabetes). Many cases can be prevented by following ‘Sick day rules’. Better access to medical care, structured diabetes education to patients and caregivers are key measures to prevent acute metabolic crisis.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Anion gap
- ARDS (Acute Respiratory Distress Syndrome)
- Cerebral Oedema
- CSII (Continuous Subcutaneous Insulin Infusion)
- Fixed Rate Intravenous Insulin Infusion (FRIII)
- Hypokalaemia
- Osmolality
- Variable Rate Intravenous Insulin Infusion (VRIII)
1 Introduction
DKA and HHS are two similar yet in many ways different metabolic emergencies of diabetes are encountered in emergency departments. Hyperglycemia, despite being the common ground for both conditions, is different in magnitude for each emergency, being more severe in HHS. Ketoacidosis is the hallmark of DKA found mostly in T1D due to absolute insulin deficiency. In HHS, ketoacidosis is nominal unless a mixed variety, which is due to residual insulin sufficient to prevent ketosis. It was thought that DKA is specific condition for T1D and HHS for T2D but this does not hold anymore. More and more cases of DKA are being reported in T2D and HHS in T1D. Similarly, the characteristic age distribution of acute hyperglycemic emergencies is not valid anymore. It is also not uncommon to find out mixture of two entities presenting in same patient.
Both of these conditions require immediate hospitalization and therefore have negative impact on the economy of a country. DKA primarily affects T1D and may be the first manifestation in up to 25% of cases (Dabelea et al. 2014; Jefferies et al. 2015; Rewers et al. 2008). More recently, EDKA is being found in T1D and T2D patient on SGLT2i (Peters et al. 2015). So there should be high index of suspicion in an unwell person with diabetes without hyperglycaemia on SGLT2i and EDKA should be ruled out. Up to 42% of DKA hospitalisations are due to readmissions for DKA within 1 year (Edge et al. 2016). It is a matter of solace that DKA mortality rates have fallen significantly in last 20 years from 7.96% to less than 1% (Umpierrez and Korytkowski 2016) Unfortunately, the mortality rates are still higher in patients over 60 years old with comorbidities, in low-income countries and in non-hospitalised patients (Otieno et al. 2006). The recent updated mortality in HHS is around 5–16% globally (Umpierrez and Korytkowski 2016). This high mortality rates necessitates early diagnosis and effective prevention programmes. Cheaper insulin should be readily available globally (Greene and Riggs 2015). The most common cause of mortality is cerebral oedema in children and young adults. On the other hand, the main causes of mortality in adults and elderly with HHS are diverse and many. The major causes are severe hypokalaemia, cardiac dysrhythmia, severe hypoglycaemia, ARDS, pneumonia, ACS (Acute Coronary Syndrome) and sepsis (Wolfsdorf et al. 2018).
Efforts should be directed to decrease hospitalization rate and acute metabolic crisis of diabetes by introducing structured diabetes education and provision of better healthcare to less developed areas. There exist some subtle differences in management protocols of DKA, EDKA and HHS patients. The purpose of this review is to provide the latest insights of epidemiology, pathophysiology, management and prevention of acute metabolic emergencies of diabetes.
2 Classification and Diagnostic Criteria
History, clinical examination, signs & symptoms and biochemical tests are required to aid diagnosis of condition. The classical triad of DKA includes hyperglycemia, ketonaemia and high anoin gap metabolic acidosis. The biochemical criterion set by JBDS, BSPED and ISPAD for diagnosis of DKA (Dhatariya 2014; Wolfsdorf et al. 2018; BSPED 2020) are as follows:
-
Ketonaemia (blood level > 3 mmol/l) or ketonuria (2+ on dipstick)
-
Hyperglycemia (>11 mmol/l) or known diabetic patient)
-
Acidosis (HCO3− < 15 mmol/l and/or venous pH <7.3)
Classification of DKA is generally based upon anion gap, HCO3, pH and cognitive status of patient.
Table 1 Classification of DKA in adults and children (BSPED 2020; Kitabchi et al. 2009; Sheikh-Ali et al. 2008; Kelly 2006).
Table 2 ADA, JBDS and AACE/ACE diagnostic criteria of DKA (Karslioglu French et al. 2019). The table is reproduced with permission.
The diagnostic criteria of DKA differs in many ways between societies. There is no consensus on all four key parameters such as ketonaemia/ketonuria, HCO3, pH and glucose values. Table 2 shows the diagnostic criteria formulated by key societies of diabetes.
JBDS criterion to classify severe DKA is slightly different and includes both physical and biochemical parameters.
Table 3 Diagnostic criteria of HHS by ADA and JBDS (Karslioglu French et al. 2019), reproduced with permission.
For DKA the prominent biochemical features are ketonemia and high anion gap acidosis. In HHS the circumstances are different as this condition is characterized by high osmolality and severe dehydration secondary to severe hyperglycemia. However in clinical practice, a mixed picture of DKA and HHS can also be encountered. It is also important to remember that some degree of acidosis in HHS can also be found due to nominal ketogenesis in some cases.
Table 4 Differences between DKA, HHS and EDKA (Rosenstock et al. 2018; Dandona et al. 2018; Mathieu et al. 2018; Blau et al. 2017; Rosenstock and Ferrannini 2015).
Euglycaemic DKA (EDKA/euDKA)
was first reported in 1973 (Munro et al. 1973). It should be suspected in any diabetes patients who is on SGLT2i with classic symptoms. Diagnosis can be made with the classical cutoff level of pH, HCO3 and ketone with normoglycaemia (Dhatariya 2016). Many studies have shown evidence that use of SGLT2i in T1D and in LADA cases have higher incidence of EDKA. In advanced T2D there are few case reports published recently (Rosenstock and Ferrannini 2015). Reduced carbohydrate intake, depleted glycogen reserve due to alcoholism, SGLT2i in T1D, reduction of insulin might precipitate EDKA.
Ketosis-Prone Diabetes (KPD)
KPD also called ‘Flatbush diabetes’ is found in certain ethnic minorities like African-Americans, Asians, sub-Saharan Africans and African-Caribbean. The genotype looks like idiopathic T1D but phenotype looks like T2D. Usually a middle aged obese man presents with DKA at diagnosis of new onset diabetes. Initial aggressive insulin therapy settles the acute stage. Subsequently, diet alone or a combination with oral hypoglycaemics can achieve glycaemic without need of insulin (Lebovitz and Banerji 2018). There are four types of classification of KPD such as ‘ADA’, ‘modified ADA’, ‘BMI based’ and ‘Aβ’ exist.
KPD classification ‘Aβ’ is based on the presence/ absence of autoantibodies and the presence/ absence of β-cell functional reserve. This is the most used and the most acceptable classification. The four subgroups are:
-
1.
A + β − (present autoantibodies but absent β-cell function)
-
2.
A + β + (present autoantibodies but present β-cell functional reserve)
-
3.
A − β − (absent autoantibodies and absent β-cell function) and
-
4.
A − β + (absent autoantibodies but present β-cell functional reserve).
A + β − and A − β − patients are immunologically and genetically distinct from each other but they share clinical characteristics of T1D with very low β-cell function. Whereas, A + β + and A − β + patients are immunologically and genetically distinct from each other but they share clinical characteristics of T2D with preserved β-cell functional reserve. Group 4 has the largest share of KPD with 76% (Balasubramanyam et al. 2006).
3 Epidemiology
DKA is no more considered as a metabolic emergency of only T1D (Bedaso et al. 2019; Jabbar et al. 2004; Takeuchi et al. 2017; Mudly et al. 2007). It is estimated that out of all DKA cases, around 34% occur in T2D (Kitabchi et al. 2009). Up to 25–40% cases of T1D present as DKA at first diagnosis (Duca et al. 2017). Apart from key precipitating factors, socio-economic factors also act as a causative factor of DKA. These are low income, limited access to health care facilities and illiteracy (Dabelea et al. 2014).
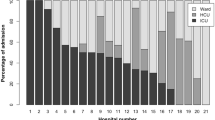
Desai et al. made a very large retrospective observational study that included 56.7 million hospitalisations during 2007–2014 with diabetes out of which 0.9% had DKA and HHS. Younger patients show (Fig. 1) highest rate of admissions with lowest mortality and the trend is reverse for older patients (Desai et al. 2019). According to a survey in USA, more than two-thirds of children presenting with HHS have T1D (Ng et al. 2020). These are mostly obese T1D and adolescent T2D. From Prospective Diabetes registry in Germany comprising 31,330 patients, DKA admission rate was 4.81/100 patient-years (Karges et al. 2015). A multinational data from 49,859 children with T1D across three registries and five nations found higher odds of DKA in females (OR 1.23), in ethnic minorities (OR 1.27) and in those with HbA1c ≥ 7.5% (OR 2.54) (Maahs et al. 2015).
Age-specific prevalence and morality of DKA and HHS (Desai et al. 2019). The figure is reproduced with permission
Table 5 Studies on SGLT2i in T1D with EDKA incidences (Rosenstock et al. 2018; Mathieu et al. 2018; Blau et al. 2017).
The hospitalization rate of HHS is less compared to DKA. According to an estimate it accounts for only 1% of diabetes related hospitalizations. In contrast to DKA, the mortality rate in HHS is considerably higher. It is 15% for HHS compared to 2–5% for DKA (Umpierrez et al. 2002). The possible explanations for this higher mortality rate for HHS are older age and presence of co-morbid conditions (Kitabchi et al. 2009).
4 Pathophysiology
4.1 DKA
Glucose homeostasis is maintained by the intricate balance of two hormones, insulin and glucagon. There are four axes (Fig. 2) which control glucose homeostasis. These are ‘Brain-islet axis’, ‘Liver-islet axis’, ‘Gut-islet axis’ and ‘Adipocytes/ myocytes-islet axis’. These axes interplay with positive and negative feedback to maintain glucose homeostasis (Röder et al. 2016).
Interplay of pancreas with brain, liver, gut, adipose and muscle tissue to maintain glucose homeostasis (Röder et al. 2016). The figure is reproduced with permission
In DKA this hormonal balance of body is tilted towards counter-regulatory hormones due to absolute insulin deficiency. Due to this shift in balance, while liver uninhibitedly keeps on producing more glucose, peripheral tissues are not able to utilize glucose from blood in the absence of insulin (Gosmanov and Kitabchi 2000). The liver is able to secrete high amounts of glucose due to presence of two metabolic pathways namely gluconeogenesis and glycogenolysis. The gluconeogenic enzymes fructose 1, 6 bisphosphatase, phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase, and pyruvate carboxylase are mainly involved. These are stimulated by an increase in the glucagon to insulin ratio and by an increase in circulating cortisol concentrations (DeFronzo and Ferrannini 1987; Stark et al. 2014). This insulin counterregulatory hormone mismatch activates hormone sensitive lipase activity which leads to increase formation of FFAs from the triglycerides (Fig. 3). This FFAs are then beta oxidised to form acetylcoenzyme A into AcAc (Acetoacetic acid) and BOHB (Beta hydroxybutyrate) in hepatic mitochondria. These are major ketone bodies, resulting ketonemia and acidosis (Dhatariya 2016; Barnett and Barnett 2003).
Pathogenesis of DKA and HHS. Reproduced with permission (Karslioglu French et al. 2019)
Ketogenesis
Conversion of FFAs into ketones in the hepatic mitochondria needs certain conditions. These are lower insulin to glucagon ratio, reduction in activity of acetyl CoA carboxylase and low levels of malonyl CoA. These eventually trigger transportation of FFAs inside mitochondria by CPT-1(Carnitine Palmitoyltransferase-1) for conversion to ketones. FFAs in hepatic mitochondria are then broken down into acetyl CoA by beta-oxidation. Two acetyl-CoA molecules are converted into acetoacetyl-CoA by enzyme thiolase. Then this acetoacetyl-CoA is converted into HMG-CoA by HMG-CoA synthase. Then HMG-CoA is converted into acetoacetate by HMG-CoA lyase. Acetoacetate then converted into either acetone through nonenzymatic decarboxylation or into BOHB by beta-hydroxybutyrate dehydrogenase. In extra-hepatic tissues acetone is either excreted via urine or exhaled and BOHB is converted into acetoacetate by beta-hydroxybutyrate dehydrogenase. This end product acetoacetate is converted back to Acetyl CoA by beta-ketoacyl-CoA transferase (Fig. 4). This way ketogenesis continues till intervention is done (Dhillon and Gupta 2019). They are preferred over glucose in absence of insulin by many peripheral tissues like brain, and skeletal muscles (Barnett and Barnett 2003; Dhillon and Gupta 2019).
Acidosis
As the concentration of Acetoacetic acid and β-hydroxybutyric acid increases in blood, they dissociates completely at physiological pH converting into acetoacetate and β-hydroxybytyrate respectively. This conversion yields hydrogen ion with each molecule which is at normal physiological state buffered by bicarbonate. In DKA the enormous amount of hydrogen ion forms due to ketogenesis. At one point bicarbonate buffering system fails and hydrogen ion concentration shoots up leading to falling blood pH and low bicarbonate (Dhatariya 2016; Barnett and Barnett 2003). The increased serum levels of glucose and ketones contributes towards osmotic diuresis and hence electrolytes disturbances and dehydration (Karslioglu French et al. 2019).
4.2 HHS
The pathogenesis of HHS differs from DKA significantly. Measurable insulin in T2D is higher than in DKA patients, which is sufficient to suppress lipolysis and ketogenesis but inadequate to regulate hepatic glucose production and promote peripheral glucose utilization. Studies have shown the half-maximal concentration of insulin for anti-lipolysis is lower than for glucose utilization by peripheral tissues (Pasquel and Umpierrez 2014; Miles et al. 1983; Umpierrez et al. 1996). The counter-regulatory hormones are high in HHS due to presence of stresses like infection, and myocardial infarction. The reason behind more severe dehydration and hyperglycemia in HHS is that it develops over several days of continued osmotic diuresis. This leads to hypernatremia, especially in older patients with renal impairment. This is worsened by inability to drink adequate water to keep up urinary losses resulting in profound dehydration. Furthermore, this hypovolemia deteriorates glomerular filtration rate as clinically shown by higher creatinine values in HHS and it eventually leads to severe hyperglycaemic state (Umpierrez et al. 2002; Kitabchi et al. 2006).
4.3 EDKA
In T2D on SGLT2i, the lower insulin to glucagon ratio stimulates lipolysis which makes around 20% enhanced lipid oxidation. This happens at the expense of markedly reduced carbohydrate oxidation, which falls by 60%. In the face of lower glucose concentrations, nonoxidative glucose disposal by glycogen synthesis and lactate release also fall by 15% (Rosenstock and Ferrannini 2015). Reduced insulin level causes reduced formation of acetyl-CoA, so inhibition of CPT-I is less. This promotes transport of FFAs into mitochondria and hence ketogenesis (Diaz-Ramos et al. 2019). BOHB levels rises by two folds in fasting and fed state. Plasma lactate level decreases to 20% which reflects reduced carbohydrate utilization. In T1D where absolute insulin deficiency prevails and if carbohydrate availability is drastically reduced then the mild ketosis would lead to ketoacidosis (Rosenstock and Ferrannini 2015) (Fig. 5).
Mechanism of development of EDKA with SGLT2i (Diaz-Ramos et al. 2019). The slide is reproduced with permission
5 Precipitating Factors
The predominant trigger for an acute DKA episode is insulin omission or non-adherence, whereas in HHS infections are most common precipitating factors. In EDKA, there is common association with the use of SGLT2i which ensures good glycaemic control that might lead to reduction in insulin dosage. These triggers higher secretion of counter-regulatory hormones in DKA, HHS and EDKA. Table 6: Precipitating factors in DKA, HHS and EDKA (Pasquel and Umpierrez 2014; Laine 2010; Adeyinka and Kondamudi 2020).
6 Clinical Presentation
DKA and EDKA evolve in hours to days but HHS develops gradually over several days to weeks. Results of a systematic review which included 24,000 children from 31 countries suggested that those who are very young and belong to ethnic minorities are more to present acutely in such manner. Other major risk factors include lean built of body, errors in diagnosis of T1D, treatment delays, infection as presenting complaint etc. Presence of T1D in family history, on the other hand, makes it unlikely that DKA would be the first presentation (Usher-Smith et al. 2011). Up to 25% patients with new-onset T1D present with DKA (Choleau et al. 2014; Jefferies et al. 2015; Usher-Smith et al. 2011).
Table 7 Features of clinical presentations of DKA, HHS and EDKA (BSPED 2020; Hardern 2003; Trence and Hirsch 2001; Hamdy 2019; Nyenwe et al. 2010; Usher-Smith et al. 2011).
In an otherwise healthy child without any family history of diabetes who presents as with urinary symptoms, it is challenging to diagnose T1D (Rafey et al. 2019). Up to 20% people present with HHS as first presentation of new-onset T2D (Pasquel and Umpierrez 2014). EDKA is difficult to diagnose without detail history and work-up as there is usually very low index of suspicion with normoglycaemia.
7 Laboratory Investigations
The laboratory investigations in DKA, HHS and EDKA are aimed primarily for diagnosis and to determine its severity. Then the next step is to identify the underlying causes, early identification of complication and monitoring of response to therapy. As acute metabolic decompensation is an emergency so just after history, clinical examination and provisional diagnosis management starts. Blood and urine samples are taken to do initial investigations without delay. Work-up for DKA and EDKA are same. Table 8 Initial and subsequent work-ups in the management of DKA and HHS (Wolfsdorf et al. 2018; Savage et al. 2011; Scott et al. 2015; Khazai and Umpierrez 2020; Gosmanov and Nimatollahi 2020; Rawla et al. 2017).
8 Differential Diagnosis
The acute hyperglycemic emergencies DKA and HHS are at the top of differential list for each other. The only biochemical feature that differentiates DKA from EDKA is serum glucose level: normal in EDKA and raised in DKA. The distinguishing feature of HHS is presence of high serum osmolality due to extremely high serum glucose levels. The hyperglycemia in HHS is generally greater than 30 mmol/l (Scott et al. 2015; Westerberg 2013).
Ketoacidosis besides being present in diabetes can also occur during starvation and alcoholism. It is important to rule out other causes of high anion gap acidosis like salicylate poisoning, methanol intoxication and lactic acidosis (Keenan et al. 2007). Since abdominal pain and vomiting episodes are often present in such patients, other etiological causes of acute abdomen like pancreatitis and gastroenteritis should also be considered in differential diagnosis (Keenan et al. 2007).
Due to presence of focal neurological deficit, HHS is very commonly confused with stroke (Umpierrez et al. 2002).
Table 9 Differential diagnosis of acute hyperglycaemic crisis (Westerberg 2013; Rawla et al. 2017; Keenan et al. 2007).
9 Management: General
Successful management of DKA, HHS and EDKA needs 5 major components to rectify as follows (Hamdy 2019):
-
1.
Correction of dehydration
-
2.
Correction of hyperglycaemia and ketoacidosis
-
3.
Correction of electrolyte abnormalities
-
4.
Identification of comorbid and precipitating factors and
-
5.
Frequent monitoring and prevention of complications
Once acute metabolic crisis of diabetes is recognized the patient needs to be hospitalized in emergency or acute medical unit or in HDU (High Dependency Unit) or in ICU depending on grading.
Table 10 The markers of severity in DKA, HHS and EDKA for HDU/ICU admission (Savage et al. 2011; Scott et al. 2015).
The markers of severity should be assessed and recorded (Table 11).
Management should start with prompt assessment of ABCDE (Airway, Breathing, Circulation, Disability-conscious level and Exposure-clinical examination) at emergency department. Acute metabolic crisis in diabetes leads to profound water and electrolyte loss due to osmotic diuresis by hyperglycaemia. In EDKA due to very nominal rise of glucose, water deficit is not profound like DKA but is significant due to ketoacidosis. Without finding the cause of acute metabolic crisis, the management is not complete. Without preceding a febrile illness or gastroenteritis, DKA in a known diabetes patient is usually due to psychiatric disorders such as eating disorders and failure of appropriately administering insulin (Wolfsdorf et al. 2018). Comparatively more aggressive fluid replacement in HHS is needed than DKA to expand intra and extra vascular volume. The purpose is to restore normal kidney perfusion, to normalize sodium concentration and osmolality. DKA usually resolves in 24 h but in HHS correction of electrolytes and osmolality takes 2–3 days. Usually HHS occurs in elderly with multiple co-morbidities, so recovery largely depends on previous functional level and precipitating factors. In EDKA if SGLT2i is suspected, it should be stopped immediately and should not restart unless another cause for DKA is found and resolved (Evans 2019).
9.1 Management: From Admission to 24–48 Hours
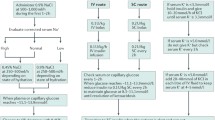
Table 12 Shows the details of management from admission onwards (Wolfsdorf et al. 2018; BSPED 2020; Savage et al. 2011; Scott et al. 2015; Evans 2019) (Fig. 6).
DKA and HHA management algorithm reproduced with permission (Cardoso et al. 2017)
9.2 Management: Acute Hyperglycaemic Crisis Due to COVID-19
The pandemic COVID-19 infection increases the risk of precipitating atypical DKA, HHS or mixed crisis and stress hyperglycaemia with ketones. The recent guideline from ABCD (Association of British Clinical Diabetologists) named ‘COVID: Diabetes’ (COncise adVice on Inpatient Diabetes) has outlined to manage COVID-19 in hyperglycaemic crisis in diabetes (ABCD 2020). This guideline is based on UK experience of COVID-19 management and will be updated further when more evidences will be available. COVID-19 infection in known or unknown people with diabetes increases the risk of acute hyperglycaemia with ketones, DKA and HHS. Poorly controlled elderly diabetes patients are more susceptible to COVID-19 and its complications. Because hyperglycaemia can subdue immunity by disrupting the normal function of WBC and other immune cells. Good glycaemic control and following sick day rules are key to reduce risk apart from taking personal protection and social distancing.
Table 13 shows COVID-19 specific management of acute hyperglycaemic crisis paraphrased from the ‘COVID: Diabetes’ guideline (ABCD 2020).
9.3 Management: Some Controversial Issues
-
0.9% NaCl vs Hartmann’s solution: In a recent RCT (Yung et al. 2017), comparing Hartmann’s solution with 0.9% NaCl in 77 children with DKA, it was observed that slightly quicker resolution of acidosis can be achieved with Hartmann’s solution in severe DKA. There was however, no difference regarding time required to shift from intravenous to subcutaneous insulin.
-
0.9%NaCl vs Ringer Lactate solution: A RCT showed no benefit from using Ringer Lactate solution compared with 0.9% NaCl in terms of pH normalization. But Ringer Lactate solution made longer time to reach blood glucose level of 14 mmol/L because lactate converts into glucose (Van Zyl et al. 2011).
-
0.9%NaCl vs Plsma-Lyte 148: The concern regarding excessive administration of normal saline in DKA is hyperchloremia which can lead to non-anion gap metabolic acidosis. Although self-limiting in nature, this hyperchloremic metabolic acidosis is now believed to have a harmful impact on multiple organs of body like kidneys, myocardium etc. (Eisenhut 2006; Kraut and Kurtz 2014). Plasma-Lyte 148 when compared to normal saline has shown to decrease occurrence of hyperchloremia (Andrew and Patrick 2018; Chua et al. 2012). A systematic review by Gershkovich et al. (Gershkovich et al. 2019) might help aid the decision regarding fluid choice in future.
-
0.9% NaCl vs Ringer Acetate solution: Though Ringer Acetate is not a popular choice but it has almost similar composition like Ringer Lactate. But its use in hepato-renal emergencies are established (Ergin et al. 2016). Figure 7 shows water shift in hyperglycaemic emergencies with different infusion fluids. The figure is reproduced with permission (Cardoso et al. 2017).
-
Infusion rate: Regarding infusion rate, rapid administration is feared to increase likelihood of cerebral edema especially in children and young adults. The JBDS guidelines therefore recommend gradual correction of fluid deficit over 48 h unless clear signs of hypovolemic shock are present (Dhatariya 2014).
-
Arterial or venous sample: the difference between venous and arterial pH is 0.02–0.15 and the difference between arterial and venous HCO3 is 1.88 mmol/L. These neither affect the diagnosis nor the treatment. But getting arterial sample is risky and painful. So venous sample is widely accepted.
-
The target with fluid administration in HHS is to achieve an hourly drop of 3–8 mOsm/kg in osmolality and 5 mmol/L in glucose. Some adjustments in fluid administration rate and solution type are required if these targets are not being met (Scott et al. 2015). These scenarios are mentioned in Table 14 (Scott et al. 2015).
ICC Intracellular compartment, ISC Interstitial compartment, IVC Intravascular compartment. Panel A: Total body water distribution in normal state; Panel B: After correction of water deficit with 5% Dextrose water shows suboptimal replenishment of IVC, ISC and excessive rehydration of ICC; Panel C: Correction with 0.9% NaCl made exclusive distribution in extracellular compartment resulting excessive hydration of IVC and ISC; Panel D: Correction with 0.45% NaCl shows replenishment similar to fluid lost from IVC, ISC and ICC. It is probably the best option; Panel E: Correction with 0.225% resulted in suboptimal replenishment of IVC, ISC but excessive hydration of ICC
9.4 Management: DKA and EDKA IN Pregnancy
DKA is an emergency during pregnancy and may cause fetal loss which is around 10–25%. The incidence rate is 1–3%. The main causes and precipitating factors are (Savage et al. 2011):
-
Starvation: accelerated maternal response ends up in DKA in women with diabetes
-
Increased flux of glucose from mother to fetus and placenta: due to increased transporter GLUT-1.
-
Higher progesterone level: induces respiratory alkalosis which results in metabolic acidosis that reduces buffering capacity.
-
Precipitating factors: UTI, hyperemesis gravidarum, new onset T1D, KPD, insulin omission, insulin pump malfunction, glucocorticoid use for inducing fetal lung maturity, use of terbutaline to prevent premature labour.
-
DKA and EDKA management is same like non-pregnant cases.
9.5 Management: Key Calculations
Table 15 shows the key calculations needed during management of acute hyperglycaemic crisis (Wolfsdorf et al. 2018).
10 Complications
The probable complications of DKA and HHS are tabulated in Table 16 (Savage et al. 2011; Scott et al. 2015; Khazai and Umpierrez 2020).
Cerebral Edema
Cerebral edema (CE)’ is rare and most feared iatrogenic complication of DKA in younger children and in newly diagnosed T1D. It is associated with high mortality and neurodisability & neurocognitive difficulties in survived cohorts. Headache, lethargy, papillary changes and seizure are key manifestation.
Risk of CE found in a study with higher plasma urea, lower arterial pCO2 and NaHCO3 therapy in DKA (Glaser et al. 2001). Interleukin-1 and 6 (IL-1 and IL-6) are the cytokines that initiate the inflammatory response accompanied by DKA. It is postulated that this IL-1 is linked with the pathogenesis of CE. NLRP3 (nucleotide-binding domain and leucine-rich repeat pyrin 3 domain) is an inflammasome which generates active form of IL-1 in response to hyperglycaemia acts as osmosensors to cause CE in DKA. It contributes to CE and infarction by making tight junctions leaky (Eisenhut 2018). Some studies have found that initial bolus of rehydration fluid and bolus insulin might have a role (Carlotti 2003).
Table 17 shows the diagnosis of cerebral edema in DKA (Wolfsdorf et al. 2018).
The management of cerebral edema is difficult and involves careful administration of fluids with strict blood pressure control and infusion of mannitol or hypertonic saline. Mannitol is administered at dose of 0.5–1 g/kg body weight. The calculated dose is administered over a period of 10–15 min and if necessary repeated after 30 min (Wolfsdorf et al. 2018). If mannitol is not available or if there is no response to mannitol, 3% hypertonic saline can be given at calculated dose of 2.5–5 ml/kg. The time for administration is again 10–15 min (Wolfsdorf et al. 2018).
Regarding mannitol versus hypertonic saline selection, controversies exist but recent data suggests lower mortality rate with mannitol (Wolfsdorf et al. 2018).
Figure 8 Pathogenesis of cerebral edema in DKA. The figure is reproduced with permission (Carlotti 2003)
A bolus of saline could expand the intracranial interstitial volume. A bolus of insulin could expand the intracerebral ICF volume (Scott et al. 2015)
.
11 Prevention
Management of acute hyperglycemic emergencies is not complete until steps are taken to prevent recurrence of future episodes. Diabetes education is an important component of prevention strategy. The education should be tailored to the individual’s requirement. This is only possible after trigger has been identified. Ideally this should be delivered by a specialized diabetes educator (Karslioglu French et al. 2019).
Proper education regarding sick day rules is essential to prevent recurrence. The important components of sick day management include education regarding hydration, glucose and ketones monitoring, continuation of basal insulin and timely contact with health care provider (Karslioglu French et al. 2019). Since the process of ketogenesis occurs in the absence or deficiency of insulin, its recurrence can be avoided. One of the major reasons for recurrence of DKA is non-compliance with insulin in teenagers of less privileged areas who are being most commonly affected. These patients can benefit from targeted community support programs (Dabelea et al. 2014).
Patient on insulin pump is at high risk of DKA in case of pump failure. Therefore one should be educated regarding its care. One should also have an emergency contact number for technical support. In case of pump failure, one might require multiple daily injections to prevent DKA as insulin reserve in body is very limited for a patient on insulin pump. Therefore one must be educated in this regard and advised to carry a reserve of long-acting insulin (Jesudoss and Murray 2016; Rodgers 2008).
12 Conclusion
DKA, EDKA and HHS are avoidable metabolic emergencies both of which can be decreased in incidences with education regarding diabetes management in sick days. The management principles are different for each condition but generally require hospitalization and intravenous fluids with electrolytes. While close monitoring during episode has decreased mortality rate, there are still some controversial areas like fluid choice for rehydration. Due to availability of updated guidelines management is much better now. The structured diabetes education and abiding by sick day rules made significant improvement in reducing the recurrences of acute metabolic crisis of diabetes.
References
ABCD (2020) COncise AdVice on Inpatient Diabetes (COVID: Diabetes): Front Door Guidance National Inpatient Diabetes COVID-19 Response Team COVID-19 Infection in People with or without Previously Recognised Diabetes Increases the Risk of the EMERGENCY States of Hyperglycaemia with Ketones, Diabetic Keto Acidosis (DKA) and Hyperosmolar Hyperglycaemic State (HHS), 9 April 2020
Adeyinka A, Kondamudi NP (2020) Hyperosmolar Hyperglycemic Nonketotic Coma (HHNC), Hyperosmolar hyperglycemic nonketotic syndrome. PubMed, Stat Pearls Publishing. www.ncbi.nlm.nih.gov/books/NBK482142/#
Andrew W, Patrick D (2018) P18 plasma-Lyte 148 vs 0.9% saline for fluid resuscition in children: electrolytic and clinical outcomes. Arch Dis Child 103(2):e1.22–e1.e1
Balasubramanyam A et al (2006) Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care 29(12):2575–2579
Barnett C, Barnett Y (2003) Ketone bodies. In: Encyclopedia of food sciences and nutrition. Academic, Amsterdam, pp 3421–3425
Bedaso A, Oltaye Z, Geja E, Ayalew M (2019) Diabetic ketoacidosis among adult patients with diabetes mellitus admitted to emergency unit of Hawassa university comprehensive specialized hospital. BMC Res Notes 12(1):137
Blau JE et al (2017) Ketoacidosis associated with SGLT2 inhibitor treatment: analysis of FAERS data. Diabetes Metab Res Rev 33(8):e2924
BSPED (2020) BSPED interim guideline for the management of children and young people under the age of 18 years with diabetic ketoacidosis, 1 January 2020
Cardoso L et al (2017) Controversies in the management of hyperglycaemic emergencies in adults with diabetes. Metabolism 68(68):43–54
Carlotti APCP (2003) Importance of timing of risk factors for cerebral oedema during therapy for diabetic ketoacidosis. Arch Dis Child 88(2):170–173
Choleau C, Maitre J, Filipovic Pierucci A, Elie C, Barat P et al (2014) Ketoacidosis at diagnosis of type 1 diabetes in French children and adolescents. Diabetes Metab 40(2):137–142. Elsevier Masson
Chua H-R et al (2012) Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care 27(2):138–145
Dabelea D, Rewers A, Stafford J, Standiford D, Lawrence J, Saydah S et al (2014) Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 133(4):e938–e945
Dandona P et al (2018) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 41(12):2552–2559
DeFronzo RA, Ferrannini E (1987) Regulation of hepatic glucose metabolism in humans. Diabetes Metab Rev 3(2):415–459
Desai R et al (2019) Temporal trends in the prevalence of diabetes decompensation (diabetic ketoacidosis and hyperosmolar hyperglycemic state) among adult patients hospitalized with diabetes mellitus: a Nationwide analysis stratified by age, gender, and race. Cureus 11(4). https://doi.org/10.7759/cureus.4353
Dhatariya K (2014) Diabetic ketoacidosis and hyperosmolar crisis in adults. Medicine 42(12):723–726
Dhatariya K (2016) Blood ketones: measurement, interpretation, limitations, and utility in the management of diabetic ketoacidosis. Rev Diabet Stud 13(4):217–225
Dhillon KK, Gupta S (2019) Biochemistry, Ketogenesis. [online] Nih.gov. Available at: https://www.ncbi.nlm.nih.gov/books/NBK493179/. Accessed 15 May 2020
Diaz-Ramos A et al (2019) Euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter-2 inhibitor use: a case report and review of the literature. Int J Emerg Med 12(1):27
Duca LM et al (2017) Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care 40(9):1249–1255
Edge JA et al (2016) Diabetic ketoacidosis in an adolescent and young adult population in the UK in 2014: a national survey comparison of management in paediatric and adult settings. Diabet Med 33(10):1352–1359
Eisenhut M (2006) Causes and effects of hyperchloremic acidosis. Crit Care 10(3):413
Eisenhut M (2018) In diabetic ketoacidosis brain injury including cerebral oedema and infarction is caused by interleukin-1. Med Hypotheses 121:44–46
Ergin B et al (2016) The role of bicarbonate precursors in balanced fluids during haemorrhagic shock with and without compromised liver function. Br J Anaesth 117(4):521–528
Evans K (2019) Diabetic ketoacidosis: update on management. Clin Med 19(5):396–398
Gershkovich B et al (2019) Choice of crystalloid fluid in the treatment of hyperglycemic emergencies: a systematic review protocol. Syst Rev 8(1):228
Glaser N, Barnett P, McCaslin I et al (2001) Risk factors for cerebral edema in children with diabetic ketoacidosis. N Engl J Med 344:264–269
Gosmanov AR, Kitabchi AE (2000) Diabetic ketoacidosis [Updated 2018 April 28]. In: Feingold KR, Anawalt B, Boyce A et al (eds) Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279146/
Gosmanov AR, Nimatollahi LR (2020) Diabetic ketoacidosis – symptoms, diagnosis and treatment | BMJ Best Practice. Bestpractice.Bmj.Com. February. https://bestpractice.bmj.com/topics/en-us/162. Accessed 15 Apr 2020
Greene JA, Riggs KR (2015) Why is there no generic insulin? Historical origins of a modern problem. N Engl J Med 372(12):1171–1175
Hamdy O (2019) Diabetic Ketoacidosis (DKA): practice essentials, background, pathophysiology. Medscape.Com. 31 May. https://emedicine.medscape.com/article/118361-overview
Handelsman Y et al (2016) American association of clinical endocrinologists and American college of endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract 22(6):753–762
Hardern RD (2003) Emergency management of diabetic ketoacidosis in adults. Emerg Med J 20(3):210–213
Jabbar A, Farooqui K, Habib A, Islam N, Haque N, Akhter J (2004) Clinical characteristics and outcomes of diabetic ketoacidosis in Pakistani adults with type 2 diabetes mellitus. Diabet Med 21(8):920–923
Jefferies C et al (2015) 15-year incidence of diabetic ketoacidosis at onset of type 1 diabetes in children from a regional setting (Auckland, New Zealand). Sci Rep 5(1):P3
Jesudoss M, Murray R (2016) A practical guide to diabetes mellitus, 7th edn. Jaypee Brothers, New Delhi
Karges B, Rosenbauer J, Holterhus PM et al (2015) Hospital admission for diabetic ketoacidosis or severe hypoglycemia in 31,330 young patients with type 1 diabetes. Eur J Endocrinol 173(3):341–350
Karslioglu French E et al (2019) Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: review of acute decompensated diabetes in adult patients. BMJ 365(1114):l1114
Keenan CR et al (2007) High risk for venous thromboembolism in diabetics with hyperosmolar state: comparison with other acute medical illnesses. J Thromb Haemost 5(6):1185–1190
Kelly A-M (2006) The case for venous rather than arterial blood gases in diabetic ketoacidosis. Emerg Med Australas 18(1):64–67
Khazai N, Umpierrez G (2020) Hyperosmolar hyperglycaemic state – symptoms, diagnosis and treatment | BMJ best practice. Beta-Bestpractice.Bmj.Com. March. https://beta-bestpractice.bmj.com/topics/en-gb/1011. Accessed 15 Apr 2020
Kitabchi AE et al (2006) Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 29(12):2739–2748
Kitabchi A, Umpierrez G, Miles J, Fisher J (2009) Hyperglycemic crises in adult patients with diabetes. Diabetes Care 32(7):1335–1343
Kraut JA, Kurtz I (2014) Treatment of acute non-anion gap metabolic acidosis. Clin Kidney J 8(1):93–99
Laine C (2010) Diabetic Ketoacidosis. Ann Intern Med 152(1):ITC1
Lebovitz HE, Banerji MA (2018) Ketosis-prone diabetes (Flatbush diabetes): an emerging worldwide clinically important entity. Curr Diab Rep 18(11):120
Maahs DM, Hermann JM, Holman N et al (2015) Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care 38(10):1876–1882
Mathieu C et al (2018) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care 41(9):1938–1946
Miles JM et al (1983) Effects of free fatty acid availability, glucagon excess, and insulin deficiency on ketone body production in postabsorptive man. J Clin Investig 71(6):1554–1561
Mudly S, Rambiritch V, Mayet L (2007) An identification of the risk factors implicated in diabetic ketoacidosis (DKA) in type 1 and type 2 diabetes mellitus. S Afr Fam Pract 49(10):15-15b
Munro JF et al (1973) Euglycaemic diabetic ketoacidosis. BMJ 2(5866):578–580
Ng S, Edge J, Timmis A (2020) Practical management of hyperglycemic hyperosmolar state (HHS) in children [Internet] [cited 6 April 2020]. Available from: http://www.a-c-d-c.org/wp-content/uploads/2012/08/Practical-Management-of-Hyperglycaemic-Hyperosmolar-State-HHS-in-children-2.pdf
Nyenwe EA et al (2010) Acidosis: the prime determinant of depressed sensorium in diabetic ketoacidosis. Diabetes Care 33(8):1837–1183
Otieno CF et al (2006) Diabetic ketoacidosis: risk factors, mechanisms and management strategies in Sub-Saharan Africa: a review. East Afr Med J 82(12). https://doi.org/10.4314/eamj.v82i12.9382
Pasquel FJ, Umpierrez GE (2014) Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 37(11):3124–3131
Peters AL et al (2015) Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care 38(9):1687–1693
Rafey MF et al (2019) Prolonged acidosis is a feature of SGLT2i-induced euglycaemic diabetic ketoacidosis. Endocrinol Diabetes Metab Case Rep 1:1–5
Rawla P et al (2017) Euglycemic diabetic ketoacidosis: a diagnostic and therapeutic dilemma. Endocrinol Diabetes Metab Case Rep 2017(1):1–4. www.ncbi.nlm.nih.gov/pmc/articles/PMC5592704/
Rewers A et al (2008) Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the search for diabetes in youth study. Pediatrics 121(5):e1258–e1266
Röder PV et al (2016) Pancreatic regulation of glucose homeostasis. Exp Mol Med 48(3):e219–e219
Rodgers J (2008) Using insulin pumps in diabetes: a guide for nurses and other health care professionals. Wiley, Chichester
Rosenstock J, Ferrannini E (2015) Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 38(9):1638–1642
Rosenstock J et al (2018) Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 41(12):2560–2569
Savage M et al (2011) Joint British diabetes societies guideline for the management of diabetic ketoacidosis. Diabet Med 28(5):508–515
Scott AR et al (2015) Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med J Br Diabet Assoc 32(6):714–724
Sheikh-Ali M et al (2008) Can serum –hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care 31(4):643–647
Stark R, Guebre-Egziabher F, Zhao X, Feriod C, Dong J, Alves T et al (2014) A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J Biol Chem 289(11):7257–7263
Takeuchi M, Kawamura T, Sato I, Kawakami K (2017) Population-based incidence of diabetic ketoacidosis in type 2 diabetes: medical claims data analysis in Japan. Pharmacoepidemiol Drug Saf 27(1):123–126
Trence DL, Hirsch IB (2001) Hyperglycemic crises in diabetes mellitus type 2. Endocrinol Metab Clin N Am 30(4):817–831
Umpierrez G, Korytkowski M (2016) Diabetic emergencies – ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol 12(4):222–232
Umpierrez GE et al (1996) Diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Am J Med Sci 311(5):225–233
Umpierrez GE et al (2002) Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectr 15(1):28–36
Usher-Smith JA et al (2011) Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ 343(1):d4092–d4092
Van Zyl DG et al (2011) Fluid management in diabetic-acidosis – Ringer’s lactate versus normal saline: a randomized controlled trial. QJM 105(4):337–343
Westerberg DP (2013) Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician 87(5):337–346
Wolfsdorf JI et al (2018) ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes 19:155–177
Yung M et al (2017) Controlled trial of Hartmann’s solution versus 0.9% saline for diabetic ketoacidosis. J Paediatr Child Health 53(1):12–17
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Muneer, M., Akbar, I. (2020). Acute Metabolic Emergencies in Diabetes: DKA, HHS and EDKA. In: Islam, M.S. (eds) Diabetes: from Research to Clinical Practice. Advances in Experimental Medicine and Biology(), vol 1307. Springer, Cham. https://doi.org/10.1007/5584_2020_545
Download citation
DOI: https://doi.org/10.1007/5584_2020_545
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51088-6
Online ISBN: 978-3-030-51089-3
eBook Packages: MedicineMedicine (R0)