Abstract
Introduction
To estimate lifetime prevalence of diabetes-related upper limb and non-acquired skin manifestations in a representative type 1 diabetes (T1D) population and to identify associations between these conditions and quality of life.
Methods
A questionnaire on these complications and measures of quality of life (World Health Organization–Five Well-Being Index [WHO-5]), depression, and diabetes-specific burden (Problem Areas in Diabetes [PAID] scale) was sent to all T1D patients in a Danish clinic (N = 583).
Results
The response rate was 68.6%. Lifetime prevalence of any upper limb soft tissue lesion was 72%; prevalence of any skin lesion was 10.5%. Frozen shoulder and vitiligo were most common upper limb and skin manifestation, at a prevalence of 53 and 9.1%, respectively. Compared to patients with no skin lesion, those with at least one skin lesion had more depression (19 vs. 33%; P < 0.01) and lower WHO-5 scores. Frozen shoulder was associated with lower WHO-5 scores (P < 0.001), more depression (29 vs. 14%; P < 0.001), and a higher PAID score (P < 0.01). A diagnosis of carpal tunnel syndrome was associated with lower WHO-5 scores (P < 0.001), a higher risk of depression (29 vs. 16%; P < 0.01), and a higher PAID score (P < 0.001).
Conclusion
Upper limb soft tissue lesions and diabetes-specific non-acquired skin lesions are very common in patients with T1D and strongly associated with impaired life quality and increased risk of depression.
Similar content being viewed by others
Introduction
Many studies describe the prevalence, pathogenesis, and optimal treatment of classical micro- and macrovascular complications in individuals with type 1 diabetes (T1D). The findings of these studies are extremely important because retinopathy, neuropathy, nephropathy, and cardiovascular diseases influence both mortality and quality of life in affected patients [1].
Although rheumatological and skin manifestations related to T1D patients have been recognized for years, less research and perhaps less clinical attention have been devoted to these complications than to macro- and microvascular complications. It is well established that in patients with diabetes there is a higher occurrence of certain specific skin lesions and non-articular conditions involving soft tissues in the upper limb, including frozen shoulder, trigger fingers, carpal tunnel syndrome, Dupuytrens contracture, and diabetic cheiroarthropathy. These conditions are typically painful and may cause functional disability [2].
A large follow-up study from the Diabetes Control and Complication Trial/Epidemiology of Interventions and Complications (DCCT/EDIC) showed that 66% of 1217 patients had at least one abnormality, for example, frozen shoulder, trigger finger, carpal tunnel syndrome, Dupuytrens contracture, or a positive prayer sign, after a mean diabetes duration of 31 years [2]. Patients with soft tissue involvement were more likely to be female and older, have longer diabetes duration, a higher mean glycated hemoglobin (HbA1c), and neuropathy and retinopathy comorbidities, although no association with nephropathy was found [2]. Other smaller studies using different diagnostic methods have also found a high prevalence of upper limb soft tissue involvement in T1D, generally confirming these associations [3,4,5,6,7,8,9].
Non-acquired skin lesions with few treatment options, such as necrobiosis lipoidica, acantosis nigricans, bullosis diabeticorum, vitiligo, and dermopathy also occur more frequently among individuals with T1D than in the general population [10,11,12].
In individuals without diabetes, rheumatological diseases [13] and chronic active skin diseases affect the quality of life [14, 15]. To date, no studies of diabetic rheumatological manifestations or diabetic skin lesions have explored associations with quality of life in patients with T1D. Thus, the aims of our study were to: (1) estimate the lifetime prevalence of rheumatological manifestations in upper limb soft tissue and selected skin conditions in a representative sample of the Danish population with T1D and (2) analyze the impact of rheumatological or skin lesions on general and diabetes-specific quality of life among patients with T1D.
Methods
Subjects
All patients with a diagnosis of T1D (International Statistical Classification of Diseases and Related Health Problems, 10th revision [ICD-10] code E10.x) attending the outpatient clinic at Copenhagen University Hospital, Hvidovre, Denmark on January 1, 2015 were identified. Patients were excluded if they: (1) no longer attended the outpatient clinic; (2) did not speak Danish; (3) had severe psychiatric illnesses, severe brain disease, or severe malignancies, or (4) were misclassified with T1D.
Study Design
In a cross-sectional survey, questionnaires with a postage-paid return envelope were sent by mail in June 2015; no reminders were subsequently sent to non-respondents. Topics included in the comprehensive questionnaire were: (1) rheumatological and skin problems; (2) quality of life; (3) insulin dosing and glucose measurements; (4) gastrointestinal problems; (5) other endocrine diseases; (6) osteoporosis, vitamin D deficiency, and exercise; and (7) hypoglycemia. Here, we report our findings on parts (1) and (2).
Questions on rheumatological conditions were adapted and translated to Danish from items used in the DCCT/EDIC follow-up survey on musculoskeletal complications [2]. These addressed symptoms and/or diagnosis, treatment, and treatment response related to frozen shoulder, carpal tunnel syndrome, and trigger finger.
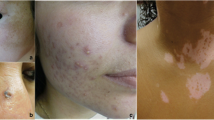
To enable patients to self-report skin problems, five color photos showing typical lesions of necrobiosis lipoidica, vitiligo, bullosis diabeticorum, acanthosis nigricrans, and diabetic dermopathy were included in the questionnaire. Lipodystrophy, a commonly acquired skin lesion in patients with T1D was not evaluated. Short descriptions of each condition were also provided. Patients were asked to separately rate each photo with a single item: “Do you have, or have you had skin lesions as pictured?” Four response options were: “I am sure”; “I think so”; “I don’t think so”; and “No, never.”
To estimate general quality of life, the World Health Organization–Five Well-Being Index (WHO-5) questionnaire, a validated 5-item measure of general well-being, was used along with a yes/no question asking whether patients had a present or past diagnosis of depression [16]. If one or more items lacked responses, the entire WHO-5 score was reported as missing. Diabetes-specific burden was evaluated with the Problem Areas in Diabetes (PAID) 20-item scale [17] and a yes/no question about any problems accepting life with diabetes so severe as to require support from someone other than diabetes healthcare providers. In the case of four or fewer missing values on the PAID scale, the individual’s mean response was used to replace the missing value. If five or more items were missing, the entire PAID was reported as missing.
The following data were extracted from hospital records for each patient as per June 1, 2015: age, sex, diabetes duration, and most recently measured body mass Index (BMI), blood pressure, HbA1c, lipids, urinary albumin-to-creatinine ratio (UACR), and vibration perception threshold (VPT).
All procedures were in accordance with the ethical standards of the Regional Ethical Committee of the Capital Region and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the survey. The Ethical Committee in the Capital Region, Copenhagen was approached and because of the nature of the project (questionnaire survey) no formal approval was needed. The data protection agency approved the project.
Definitions
Our observational study precluded any clinical examination, and we defined upper limb soft tissue involvement as follows. Patient reports of a healthcare professional (HCP) having diagnosed frozen shoulder, carpal tunnel syndrome, or trigger finger resulted in a “clear” diagnosis of these conditions. Patient reports of general shoulder pain, pain when trying to sleep, stiffness, limited joint mobility, or difficulty using the shoulder for normal daily activities resulted in a “likely” diagnosis of frozen shoulder. Similarly, patient reports of hand numbness, tingling fingers waking the patient at night, or loss of sensation or weakness in hands resulted in a likely diagnosis of carpal tunnel syndrome, and reports of a finger catching or locking in a bent position that straightened by itself or required the patient to straighten it, a finger locking in a position from which the patient was unable to straighten it, or a popping or clicking sensation while bending a finger resulted in a likely diagnosis of trigger finger. We defined skin manifestations as occurring if patients responded either “I am sure” or “I think so” to the related survey item.
Statistical Analysis
Baseline data for respondents and non-respondents were compared using Students t test, the Mann–Whitney U test, and the χ2 test, depending on the data distribution and format. The prevalence of any skin lesion, of any upper limb soft tissue manifestation, of treatment, or depression, or severe difficulty in accepting diabetes was expressed as a percentage of the total cohort of respondents. Regression models were used to assess the association between independent variables (upper limb soft tissue involvement, skin lesions, age, gender, diabetes duration and HbA1c) and quality of life measures, with multiple regression analyzes used for WHO-5 and PAID scores and binomial logistic regression used for dichotomous depression and difficulty in accepting diabetes variables. All analysis was performed using SPSS Statistics version 22 (IBM Corp., Armonk, NY, USA).
Results
Identification of Patients
An initial cohort of 641 patients with T1D were identified. After 58 patients were excluded for not fulfilling the inclusion criteria, the study population consisted of 583 patients (Fig. 1). Of these, 399 responded to the questionnaire, yielding a response rate of 68.6%.
Clinical Baseline Data
Respondents were significantly older than non-respondents (46.0 ± 13.2 vs. 39.8 ± 12.4 years; P < 0.001) and comprised proportionally more females than males (53 vs. 38%; P < 0.05); they also had lower HbA1c (7.6 ± 1.2% [59 ± 13 mmol/mol] vs. 8.2 ± 1.5% [66 ± 17 mmol/mol]; P < 0.001), lower low-density lipoprotein cholesterol (2.3 ± 0.7 vs. 2.5 ± 0.8 mmol/l; P < 0.05), and higher high-density lipoprotein cholesterol (1.8 ± 0.5 vs. 1.6 ± 0.4 mmol/l; P < 0.001) than non-respondents (Table 1). Respondents also had a non-significant longer diabetes duration than non-respondents (20.8 ± 12.9 vs. 18.9 ± 11.8 years; P = 0.07). BMI, total cholesterol, blood pressure, UACR, and VPT were similar across groups.
Rheumatological Manifestations in Soft Tissue
Lifetime prevalence of a clear frozen shoulder diagnosis was reported by 18% (n = 72) of respondents, with an additional 35% (n = 140) of respondents reporting a likely diagnosis (Table 2). Of the affected individuals who responded, 27% (n = 108) had been treated with physiotherapy and 21% (n = 84) had used analgesics; other treatments were less frequent. Clear and likely diagnoses of carpal tunnel syndrome were reported by 11% (n = 44) and 37% (n = 148) of respondents, respectively. Surgical treatment for carpal tunnel syndrome was reported by 7% of respondents (n = 28 with a clear or likely diagnosis). Clear and likely diagnoses of trigger finger were reported by 14% (n = 56) and 16% (n = 64) of respondents, respectively, with the number of affected fingers varying from one to ten (median 4). Among the respondents, 10% (n = 40) reported having had undergone surgical treatment; other treatment modalities were less frequent. The overall lifetime prevalence of rheumatological manifestations in upper limb soft tissue was 72%, with 30% of respondents reporting a clear diagnosis of frozen shoulder, carpal tunnel syndrome, or trigger finger and an additional 42% reporting a likely diagnosis of at least one of these conditions.
In the logistic regression analysis, age (odds ratio [OR] 1.028; 95% confidence interval [CI] 1.009, 1.048; P < 0.005), diabetes duration (OR 1.022; 95% CI 1.001, 1.004; P < 0.05), and female gender (OR 2.539; 95% CI 1.591, 4.054; P < 0.001) were independently associated with a clear or likely diagnosis of at least one upper limb soft tissue condition. HbA1c, UACR, and VPT were not associated with these conditions.
Skin Manifestations
Among respondents, 4.3% (n = 17) reported having had necrobiosis lipoidica, 9.1% (n = 36) had had vitiligo, and 4.8% (n = 19) had had dermopathy; other surveyed skin manifestations occurred less frequently (Table 3). One or more diabetes-related skin manifestations were reported by 10.5% (n = 42) of patients. Those with and without reported skin manifestations were similar in terms of age, gender distribution, BMI, VPT, and UACR. However, patients reporting skin manifestations had significantly longer diabetes duration (25 ± 17 vs. 20 ± 12; P < 0.05) and a tendency toward higher HbA1c (7.8 ± 1.1% [62 ± 12 mmol/mol] vs. 7.6 ± 1.2% [59 ± 13 mmol/mol]; P = 0.11).
Quality of Life
Among respondents, 21% (n = 84) reported having been diagnosed with depression by a HCP; of these, 52% (n = 44) had been treated with counseling by their general practitioner, 55% (n = 46) had been counseled by a psychiatrist, and 62% (n = 52) had been treated with antidepressant medicine. A WHO-5 score of < 50, indicating low well-being with increased risk of stress or depression (higher WHO-5 indicates better well-being), was identified in 22% (n = 88) of all respondents. Among those with current or past depression, 40% scored < 50 on the WHO-5, compared to 16% of those who had never been diagnosed with depression (P < 0.001).
In total, 91% of respondents completed all 20 PAID items. Problems accepting life with diabetes to a degree that required help from someone other than diabetes HCPs were reported by 23% of respondents. A total PAID score of > 40, indicating greater diabetes-related problems (lower PAID score indicates fewer diabetes-related problems), was identified in 32% (n = 128) of respondents completing the entire scale.
Associations Between Manifestations and Quality of Life
Skin Lesions and Quality of Life
For each of the five included skin lesions, no association was observed with WHO-5 scores or depression, nor were more diabetes-specific problems indicated by higher PAID scores found. However, patients with necrobiosis lipoidica and vitiligo reported a greater difficulty accepting life with diabetes than did patients without these conditions. WHO-5 scores were significantly lower for the entire cohort of patients with at least one of the diabetes-related skin manifestations. In addition, depression was more common among patients reporting at least one diabetes-related skin lesion than among those reporting no skin lesions (33 vs. 19%; P < 0.01). In contrast, among patients with and without skin lesions, diabetes-specific well-being measured by the PAID score and by the single item on acceptance of diabetes was similar.
Rheumatological Soft Tissue Manifestations and Quality of Life
Patients with a clear or likely diagnosis of frozen shoulder had significantly lower WHO-5 scores than those without shoulder problems (59 ± 22 vs. 66 ± 18; P < 0.001), as well as increased rates of depression (29 vs 14%; P < 0.001). Compared to patients without shoulder problems, those with shoulder problems were associated with a higher PAID score (39 ± 16 vs. 35 ± 13; P < 0.01) and a non-significant tendency to have ever had greater acceptance problems with diabetes (27 vs. 19%, P = 0.09). WHO-5 scores were significantly lower in patients with a clear or likely diagnosis of carpal tunnel syndrome than in those without carpal tunnel syndrome (57 ± 22 vs. 67 ± 17; P < 0.001) and depression was more common (29 vs. 16%; P < 0.01). Patients with carpal tunnel syndrome had a higher average PAID score than those without carpal tunnel syndrome (39 ± 15 vs. 34 ± 14; P < 0.001) but had not had more difficulty accepting diabetes (27 vs. 20%; P = 0.20). No significant differences were found between respondents with and without a diagnosis of trigger finger on any measure of general or diabetes-specific well-being (see Fig. 2).
General and diabetes-specific quality of life for patients with and without upper limb musculoskeletal/joint manifestations. Mean World Health Organization–Five Well-Being Index (WHO-5) score (higher indicates better well-being), percentage of respondents with a diagnosis of depression, Problem Areas in Diabetes scale score (PAID scale; lower score is better, indicating fewer diabetes-related problems), and percentage of respondents indicating difficulty accepting disease are shown for patients with a clear or likely diagnosis of frozen shoulder, carpal tunnel syndrome, trigger finger, or any of these upper limb manifestations (blue bar) and patients without a clear or likely diagnosis of these conditions (red bar). Symbols above bars indicate a significant difference between the two patient groups at: *P < 0.0001; §P < 0.001; ^P < 0.01; #P < 0.05
General well-being was worse (lower WHO-5 score) among patients with a clear diagnosis of any upper limb soft tissue involvement than among those without this diagnosis (WHO-5 score: 59 ± 24 vs. 63 ± 19, P < 0.01), and significantly more patients with a clear diagnosis of any upper limb soft tissue involvement reported a diagnosis of depression (P < 0.05). When patients with a likely diagnosis of any upper limb soft tissue involvement were also included in the comparison, the differences were even more pronounced. However, PAID scores and the percentage of patients with difficulty accepting diabetes were similar for respondents with and without a clear diagnosis of upper limb soft tissue involvement.
Overall Analysis of Risk for Quality of Life Impairment
Regression models were used to assess the association between independent variables (upper limb soft tissue involvement, skin lesions, age, gender, diabetes duration, and HbA1c) and quality of life measures. In a multivariate regression model, a lower WHO-5 score was positively associated with younger age (P < 0.001) and frozen shoulder or carpal tunnel syndrome (P < 0.005 for both). Other factors did not explain additional variance in the WHO-5 scores. A binominal logistic regression was performed to estimate the effects of age, diabetes duration, gender, HbA1c, at least one skin lesion, frozen shoulder, carpal tunnel syndrome, and trigger finger on the likelihood that participants had depression. The logistic regression model was statistically significant and explained 10% of the variance in depression (Nagelkerke R2). Any skin lesions (P < 0.05) and frozen shoulder (P < 0.01) increased the risk of depression, while female gender approached significance (P = 0.054). A multivariate regression model demonstrated independent significant risks of a higher PAID score for younger age (P < 0.05), shorter diabetes duration (P < 0.005), female gender (P < 0.01), high HbA1c (P < 0.05), frozen shoulder (P < 0.01), and carpal tunnel syndrome (P < 0.05). Skin lesions and trigger finger were not associated with higher PAID scores. No parameters were associated with severe problems accepting diabetes.
Discussion
In this study, we report on upper limb soft tissue conditions and non-acquired skin lesions associated with T1D and their associations with quality of life in a Danish representative cohort. The lifetime prevalence of at least one upper limb or skin manifestation was reported to be 72 and 10%, respectively, indicating that these complications appeared frequently and were highly associated with impaired quality of life and higher rates of depression. To our knowledge, this issue has not been addressed in a previous study.
In general, the prevalence of soft tissue and skin lesions in the TD1 patient population varies across studies, which can likely be attributed to differing methodologies. First, some studies have reported point prevalence and others lifetime prevalence; for most manifestations, the latter will be greater. In addition, prevalence varies across study populations. For example, some studies include only patients with long diabetes duration. Finally, the method used for the detection of lesions (e.g., questionnaires vs. clinical examinations) will also affect the results. However, despite these variations, both upper limb impairment and skin lesions are consistently reported as being highly prevalent among individuals with T1D, compared to individuals without diabetes.
In the study reported here, lifetime prevalence was investigated by surveying all T1D patients attending a large clinic, and our finding that 72% of respondents had had frozen shoulder, carpal tunnel, or trigger finger, or a combination of these, is comparable to prevalences reported in other studies. In the DCCT/EDIC follow-up study, clinical exams identified 66% of patients as having conditions that also included Dupuytrens contracture [2]. In a recent Swedish study in patients with diabetes of > 20 years duration, 79% had current or previous upper limb impairment and only 21% had no symptoms or prior surgery [5]. In a smaller Norwegian study of individuals with T1D for > 45 years, > 90% of individuals had been affected [6].
In our study, frozen shoulder was the single most frequent condition, with 53% of respondents having a clear or likely diagnosis. This result is consistent with those of other studies reporting a lifetime prevalence of frozen shoulder of 10.3–76% [2, 3, 5,6,7].
A clear or likely diagnosis of carpal tunnel syndrome was reported by 48% of respondents in our study. In the DCCT/EDIC follow-up, lifetime prevalence was 30% [2]. In recent smaller studies, lifetime prevalence was 21–37% [4, 6, 18]. In a study of patients with diabetes of > 20 years duration, 25% had received surgery for carpal tunnel syndrome, and 47%, including some patients who underwent surgery, had hand paresthesia [5]. In our cohort, only 7% of respondents had undergone surgery for carpal tunnel syndrome. The differences are likely due to our broader study population, but underdiagnoses could explain the difference between clear and likely diagnoses in our study.
We identified a lifetime trigger finger prevalence of 30%, which is similar to that reported in other studies of 20–42% [2, 5, 6, 9] of T1D patients.
In our study, patients with upper limb soft tissue lesions were older, more likely to be female, and more likely to have longer diabetes duration than patients without these manifestations, whereas glycemic control measured by the most recent HbA1c did not differ between those with and without upper limb soft tissue lesions. Our respondents as a whole were also generally older, more likely to be female, and more likely to have longer diabetes duration, which could have led to an overestimation of true prevalence of these lesions. However, our respondents also had significantly better glycemic control than reported in other studies, which is associated with reduced risk for upper limb manifestations in most studies.
Several other studies have identified an association between soft tissue upper limb lesions and long diabetes duration [2,3,4,5] and high HbA1c [2, 5,6,7, 9] and inconsistent associations between these lesions and age [2, 3, 6], female gender [2, 5, 6], and microvascular complications [2]. Surprisingly, in our study, patients with upper limb soft tissue lesions had glycemic control similar to that of patients without lesions. This contradicts many other studies and may result from our point measurement of HbA1c, as opposed to using historical values to which we did not have access. Furthermore, in our study, patients uniformly had acceptable to good metabolic control. However, we found clear positive correlations between age and duration of diabetes, which may reflect total glycemic burden better than point HbA1c values.
Different skin diseases have different manifestations and etiology, but all are likely related in some way to the autoimmune and/or hyperglycemic condition of individuals with T1D. Necrobiosis lipoidica is a chronic condition of unknown etiology that presents clinically as slowly enlarging areas of sharply demarcated brownish/yellowish plaques, often with central atrophy and most often located in the pretibial area. It has been previously estimated that 0.3% of T1D patients will develop this condition, but > 90% of individuals with this condition have diabetes and they are predominantly female [19]. A recent study from the German–Austrian register DPV revealed that among 64,133 individuals with T1D aged 0–25 years with a mean HbA1c of 7.9% (63mmol/mol), a diagnosis of necrobiosis lipoidica was present in 0.25% and was strongly positively correlated with HbA1c, duration of diabetes, presence of celiac disease, higher weight-adjusted insulin requirements, female gender, and smoking, but not with age, BMI, retinopathy/microalbuminuria, or use of insulin analogs [20]. These data contrast with our findings among adults showing that there was lifetime prevalence of necrobiosis lipoidica of > 4% . The prevalence of vitiligo in the general population is estimated at 0.5–1.0%, which is much lower than the 9.1% we found [21]. An association between vitiligo and autoimmune diseases exists, but the pathogenesis of vitiligo has not yet been fully explained. Furthermore, the evidence base for vitiligo treatment is currently inadequate, and no cure has yet been developed. In T1D patients, vitiligo has been found to be 10- to 20-fold more prevalent than in the patients in our study [21]. In a 2010 survey, over one-half of respondents felt that vitiligo moderately or severely affected their quality of life [22], which was more pronounced than in our study. This difference likely arises from differences in the severity of vitiligo among members of the Vitiligo Society [22] and in our cohort.
In the entire study population, the lifetime prevalence of depression was 21%. The prevalence of depression is typically two- to three-fold higher among those with T1D than in the general population and higher still when self-reported [23]. We found that there were twice as many reports of depression among patients with a clear current or past diagnosis of a rheumatological problem than among patients without these conditions. Accordingly, a WHO-5 score of ≤ 50, indicating high risk of depression or stress, was seen in more patients with upper limb problems than in patients without them. We have no previous data from studies of quality of life among T1D patients with rheumatological disease. However, a comparison of healthy people and patients without diabetes who had chronic rheumatological diseases, such as rheumatoid arthritis, fibromyalgia syndrome, and ankylosing spondylitis, showed a significant impairment in quality of life in the latter group [13].
In our study, patients with rheumatological problems also reported significantly worse diabetic-specific distress, with the PAID scores indicating that they had more trouble living with their diabetes than did patients without rheumatological disease. The associations were only seen for the most serious and comprehensive rheumatological manifestations, such as frozen shoulder and carpal tunnel syndrome, and not with the typically less disabling and painful trigger finger.
Our study demonstrated that current or past diabetes-related skin lesions are associated with reduced general well-being and higher rates of depression. Reduced general well-being and higher rates of depression have also been demonstrated in people without diabetes who had other chronic skin lesions, although very different methods and small samples were used for the assessment. Patients with atopic dermatitis have a similarly reduced quality of life [13], and patients suffering from psoriasis are more likely to suffer from depression when advanced disease is present [15].
Strengths and Limitations
The patients included in our large study population met well-defined inclusion and exclusion criteria. The very high response rate supports the assumption that our population was representative of the typical Danish outpatient with T1D, although we excluded a few patients who did not speak Danish and/or had a severe illness. However, our study also has several limitations. First, it is based on questionnaire data, and patients neither underwent a physical examination nor did we confirm musculoskeletal or skin diagnoses in their medical records. Compared to non-respondents, more respondents were female and older, and we may have overestimated the true prevalence of rheumatological lesions. However, respondents also had lower HbA1c levels, which may counter any overestimation effect. Secondly, it would have been of value for this study to compare prevalence data to a Danish control group without diabetes, but several other studies have previously included control groups. Further, the determination of impaired general and diabetes-specific quality of life could have been improved by the use of a broader panel of questionnaires.
Conclusion
In a large cohort that included the majority of T1D patients attending a diabetes clinic, a very high lifetime prevalence of upper limb musculoskeletal/joint disorders was observed, with 72% of patients having either symptoms or a clear diagnosis. Also, five specific skin lesions were seen frequently, with a lifetime prevalence of approximately 10%. We demonstrated for the first time that these complications were strongly associated with decreased general quality of life and depression. Previously, other authors have advocated for more focus on rheumatological problems in diabetes treatment. Based on our findings, we reiterate and expand that recommendation to include diabetes-related musculoskeletal and skin lesions, as well as quality of life, as part of routine screening for late complications of diabetes. Most rheumatological conditions are treatable and, in general, recognition of patients’ symptoms is extremely valuable even for non-acquired skin lesions that can be much more difficult to manage.
References
Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME, The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life. Diabetes Care. 2013;36:3131–8.
Larkin ME, Barnie A, Braffett BH, et al. Musculoskeletal complications in type 1 diabetes. Diabetes Care. 2014;37:1863–9.
Arkkila PE, Kantola IM, Viikari JS, Rönnemaa T. Shoulder capsulitis in type I and II diabetic patients: association with diabetic complications and related diseases. Ann Rheum Dis. 1996;55:907–14.
Raje YR, Cracknell G, Davoren PM. Frequency of hand and shoulder symptoms in patients with Type 1 diabetes. Diabet Med. 2015;32:968–71.
Gutefeldt K, Hedman CA, Thyberg ISM, Bachrach-Lindström M, Arnqvist HJ, Spångeus A (2017) Upper extremity impairments in type 1 diabetes with long duration; common problems with great impact on daily life. Disabil Rehabil 5:1–8.
Holte KB, Juel NG, Brox JI, et al. Hand, shoulder and back stiffness in long-term type 1 diabetes; cross-sectional association with skin collagen advanced glycation end-products. The Dialong study. J Diabetes Complications. 2017;31:1408–14.
Juel NG, Brox JI, Brunborg C, Holte KB, Berg TJ. Very high prevalence of frozen shoulder in patients with type 1 diabetes of ≥ 45 years’ duration: the Dialong Shoulder Study. Arch Phys Med Rehabil. 2017;98:1551–9.
Thomas SJ, McDougall C, Brown IDM, et al. Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. J Shoulder Elb Surg. 2007;16:748–51.
Ramchurn N, Mashamba C, Leitch E, et al. Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med. 2009;20:718–21.
Duff M, Demidova O, Blackburn S, Shubrook J. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40–8.
De Macedo GMC, Nunes S, Barreto T. Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetol Metab Syndr. 2016;8:1–8.
Lima AL, Illing T, Schliemann S, Elsner P. Cutaneous manifestations of diabetes mellitus: a review. Am J Clin Dermatol. 2017;18:541–53.
Ovayolu N, Ovayolu O, Karadag G. Health-related quality of life in ankylosing spondylitis, fibromyalgia syndrome, and rheumatoid arthritis: a comparison with a selected sample of healthy individuals. Clin Rheumatol. 2011;30:655–64.
Misery L, Seneschal J, Reguiai Z, et al. Patient burden is associated with alterations in quality of life in adult patients with atopic dermatitis: results from the ECLA study. Acta Derm Venereol. 2018;98:713–4.
Mahmutovic J, Zukic M, Pasalic A, Brankovic S, Jaganjac A, Katana B. Correlation between quality of life and depression among persons suffering from psoriasis. Med Arch. 2017;71:341–6.
Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84:167–76.
Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–60.
Singh R, Gamble G, Cundy T. Lifetime risk of symptomatic carpal tunnel syndrome in Type 1 diabetes. Diabet Med. 2005;22:625–30.
Murphy-Chutorian B, Han G, Cohen SR. Dermatologic manifestations of diabetes mellitus. A review. Endocrinol Metab Clin North Am. 2013;42:869–98.
Hammer E, Lilienthal E, Hofer SE, Schulz S, Bollow E, Holl RW. Risk factors for necrobiosis lipoidica in Type 1 diabetes mellitus. Diabet Med. 2017;34:86–92.
Ezzedine K, Medicina D. Seminar vitiligo. Lancet. 2015;6736:1–11.
Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: A survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736–9.
Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol. 2015;3:450–60.
Acknowledgements
We would like to thank all the patients for taking the time to answer the questionnaire, Beatriz Rodriques Reino for help with data management, Lars Krag Møller for establishing the database, and Jennifer Green for the language editing assistance.
Funding
No funding or sponsorship was received for this study or the publication of this article. The article processing charges were funded by Steno Diabetes Center Copenhagen.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Kirsten Nørgaard and Urd Kielgast have nothing to disclose.
Compliance with Ethics Guidelines
All procedures were in accordance with the ethical standards of the Regional Ethical Committee of the Capital Region and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the survey. The Ethical Committee in the Capital Region, Copenhagen was approached and because of the nature of the project (questionnaire survey) no formal approval was needed. The data protection agency approved the project.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to: https://doi.org/10.6084/m9.figshare.7694639
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Nørgaard, K., Kielgast, U. Quality of Life is Markedly Impaired by Rheumatological and Skin Manifestations in Patients with Type 1 Diabetes: A Questionnaire Survey. Diabetes Ther 10, 635–647 (2019). https://doi.org/10.1007/s13300-019-0587-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-0587-5