Abstract
Compositional gradient can be described as changes in the composition of components both vertically and horizontally in a hydrocarbon reservoir. In the present work, two-dimensional compositional gradient in multi-component gas and oil mixtures is modeled. A thermodynamic model is developed based on molecular diffusion coefficients in mass diffusive flux. A combination of Sigmund and Bird correlations is considered to estimate molecular diffusion coefficients for gas mixtures. Implementing this comprehensive developed model on a gas condensate sample shows interesting differences not only in trends of component compositions but also in gradient magnitude. A real set of data from a supergiant gas condensate field is used to validate the model. It is perceived that the developed model reduces relative absolute errors to about 50%. In the next step, a comprehensive study was conducted to understand the cross effects of molecular diffusion and natural convection in gas condensate and volatile oil samples. Gas and oil samples are selected to investigate if natural convection has the same effects in different samples. It is observed that increase in natural convection causes to reduce horizontal and vertical composition gradients. This effect is more significant in gas reservoir, as methane composition varies by more than 5.2 mol% diagonally in gas condensate sample, whereas this value is about 0.45 mol% in volatile oil sample. Lower density and higher bulk velocity in gas sample cause more disturbances in flow streams of gas mixture. Evaluation of the developed model shows that the model is reliable for reservoir studies and management programs.
Similar content being viewed by others
Introduction
Lateral and vertical variation in the composition of reservoir fluid will be expectable due to non-uniformity of pressure and temperature through a reservoir. Pressure and temperature gradients are the most well-known reasons of the compositional gradient, such that in some cases, their effect on compositional distribution is significant (Hoier and Whitson 2001). Prediction of compositional variation allows reservoir engineers to have an appropriate evaluation of hydrocarbon-in-place and phase behavior of reservoir fluid in addition to the suitable design of well-head equipment. In other words, it will prepare a suitable initialization for reservoir simulations. Regarding this point, numerous researches have been conducted to modeling this phenomenon that most of them have focused on vertical compositional gradient. These researches are led to propose isothermal and non-isothermal models based on equilibrium and irreversible thermodynamics, respectively (Whitson et al. 1994; Kempers 2001; Hasse 1962). Isothermal model based on equilibrium thermodynamics was introduced by Gibbs (Whitson et al. 1994). Using this model, Hoier and Whitson published a comprehensive study to predict the compositional gradient for different types of hydrocarbon reservoirs. They investigated that near-critical and volatile oil samples have more significant gradient than black oil and slightly volatile oil (Hoier and Whitson 2001). Literature reviews show that there are some reservoirs with considerable temperature gradient which causes non-equilibrium conditions. This leads to having some errors in predicting the compositional gradient based on the isothermal model. Thus, various forms of non-isothermal models based on irreversible thermodynamic were proposed, among them, Hasse (1962), Kempers (2001), Da Silva and Belery (1989) and Shukla and Firoozabadi (1998) are more referable. The main difference between non-isothermal models is regarding the mechanism used to calculate thermal diffusion factor. The first two models utilize enthalpy to evaluate this factor while the last two apply energy of transport to thermal diffusion factor based on kinetic theory of gases. Both isothermal and non-isothermal models are the main applicable models which are found in commercial phase behavior simulators such as PVTi (2009), PVTsim (2016) and Winprop (2013). The common feature of these models is to predict vertical variation, while also lateral gradient in composition due to convection has been reported in some cases (Montel and Gouel 1985). It is expected that the cross effects caused by gravity and convection could have noticeable effect on the distribution of compositions in different locations of reservoir. In addition, tracing of composition variation in horizontal direction could help reservoir engineers to recognize faults or barriers in reservoir rocks. Therefore, two-dimensional (2D) prediction of the compositional gradient is useful to have a reliable scheme of reservoir and its fluid phase behavior.
There are only handful of studies on 2D modeling of compositional gradient, which among them, series publications of Firoozabadi et al. are more noteworthy (Shukla and Firoozabadi 1998; Firoozabadi and Dindoruk 1995; Firoozabadi et al. 2000; Ghorayeb and Firoozabadi 2000; Riley and Firoozabadi 1998). In these studies, the main focus is on thermal diffusion and convection coupled with molecular diffusion. Shukla and Firoozabadi were presented a model to predict thermal diffusion coefficient in binary mixtures based on irreversible thermodynamic (Shukla and Firoozabadi 1998). This model is categorized in dynamic models according to its relation with energy of viscous flow. They compared the results of their model with other available models such as Hass and Kempers [static models based on enthalpy and internal energy (Kempers 2001; Hasse 1962)] and demonstrated that this new model was more reliable than others. Riley and Firoozabadi studied on compositional gradient in a binary single-phase system by coupling the effects of natural convection, due to both thermal and compositional gradients (Firoozabadi 1999), and diffusion (Riley and Firoozabadi 1998). They showed that by increasing in permeability, compositional gradients were decreased with a rate proportional to inverse of permeability. Firooazabadi and Ghorayeb completed Shukla’s study by deriving a theoretical model for thermal diffusion coefficients in ideal and non-ideal multi-component mixtures (Firoozabadi et al. 2000). They mentioned that in non-ideal mixtures, dependency of thermal diffusion coefficients on molecular diffusion is more significant than in binary mixtures. Following the previous studies, Nasrabadi and Ghorayeb implemented the model proposed by Ghorayeb and Firoozabadi (2000) to perform initialization in a 2D cross section for a binary mixture and a multi-component reservoir fluid (Nasrabadi and Ghorayeb 2006). Their results showed a strong effect of natural convection on component distributions in gas and oil phases.
Referring to model proposed by Ghorayeb and Firoozabadi (2000) and henceforth known as Ghorayeb model, molecular diffusion coefficients in gas samples are estimated using Kooijman–Taylor correlation (Kooijman and Taylor 1991) which is proposed for liquid mixtures (Ghorayeb and Firoozabadi 2000; Nasrabadi and Ghorayeb 2006), while generally, molecular diffusion coefficients of gases are almost greater about one order of magnitude than molecular diffusion coefficients of liquids. It is expected that changing value of diffusion coefficient could have a considerable effect on the prediction of the compositional gradient and also distribution of components in gas reservoir. In addition, comprehensive 2D study on the compositional gradient is performed in gas condensate reservoirs. Accordingly, principle objectives of this study are briefly described in three parts. In part 1, to illustrate the importance of using appropriate correlations and the effect of molecular diffusion coefficients in predicting compositional gradient, Ghorayeb model is developed using a set of correlations proposed for gas mixtures. Then for a same gas sample, compositional gradients predicted from this developed model and Ghorayeb model are compared to investigate the main differences. In part 2, validity of the developed model is checked for a gas condensate system through a comprehensive study on the effect of natural convection and its cross effects on 2D prediction of compositional gradient in a huge gas condensate reservoir. In part 3, applicability of the developed model on other reservoir fluids is verified through a comprehensive study of cross effects in a volatile oil sample and the results are compared with those obtained from gas condensate.
For these purposes, first the governing equations are presented, then field data and their conditions are discussed briefly and finally results are reported and analyzed.
Mathematical modeling: governing equations and boundary conditions
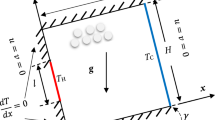
For implementing the mathematical model, a rectangular area with width w and height h is selected as porous media. A simple schematic and classification of regions based on temperature condition are shown in Fig. 1.
For this purpose, there are some main assumptions:
-
Porous media geometry is considered to be bounded by an impervious rock with constant horizontal and vertical temperature gradients.
-
No fluid flows across the outer boundaries.
-
Permeability and porosity are considered as average values in selected geometry.
To predict compositional gradient, total diffusion equation includes mass diffusion and convection should be solved in two dimensions. Conservation equation in steady-state condition will be as follows (Firoozabadi 1999):
where c, \(\vec {V}\), and \(\overrightarrow {{J_i}}\) are total molar density, bulk velocity and molar diffusive flux of component i, respectively. With respect to Darcy’s law, bulk velocity is estimated as follows:
where k and φ are rock characteristics including permeability and porosity, respectively. By replacing Eqs. (2) in (1):
Diffusive flux of component i is defined as follows (Ghorayeb and Firoozabadi 2000a, b):
In this equation, \({\mathbf{D}}_{{{\mathbf{ij}}}}^{{\mathbf{M}}}\), \({\mathbf{D}}_{{\mathbf{i}}}^{{\mathbf{P}}}\) and \({\mathbf{D}}_{{\mathbf{i}}}^{{\mathbf{T}}}\) are molecular diffusion, pressure diffusion and thermal diffusion coefficients, respectively.
There are different methods for estimating each element of the multi-component diffusion coefficients matrix, \({\mathbf{D}}_{{{\mathbf{ij}}}}^{{\mathbf{M}}}\). In this study, a correlation published by Sigmund has been implied for estimating these coefficients in gas mixtures (Sigmund 1976a, b). Sigmund has related elements of \({\mathbf{D}}_{{{\mathbf{ij}}}}^{{\mathbf{M}}}\) with mutual diffusivity, Dij, and mole fractions yi by Stefan–Maxwell relations. For a mixture containing n + 1 species, this relation is written as
It was shown by Toor et al. (1965) that diffusive flux equation could be written in form of matrix notation:
Then elements of [D] will be estimated by inverting the matrix [P] = [D]−1 such that with constant c:
The elements of [P] are given by Toor et al. (1965):
where Dik are mutual diffusivities.
There are several methods for estimating mutual diffusivities. In this study, at first, diffusivities are calculated by Bird–Slattery relation (Bird et al. 2002), Eq. (10), in low pressure and then they are corrected for reservoir condition with Eq. (11) which is presented by Sigmund (1976):
in which, \((\rho {D_{ij}})^\circ\) is zero pressure limit of the density–diffusivity product and \(\rho {D_{ij}}\) is the corrected value for reservoir pressure with the effect of reduced density. The calculated mutual diffusivities are replaced in Eqs. (8) and (9) to estimate multi-component diffusion coefficients. From now on, this combination of Sigmund and Bird correlations is known as developed model.
Refer to relations proposed by Ghorayeb et al. (Ghorayeb and Firoozabadi 2000), Eqs. (12) and (13) are utilized to estimate pressure diffusion and thermal diffusion coefficients, respectively:
The parameters \({a_{in}}\), \({D_{in}}\) and \({k_{Ti}}\) are calculated from the following equations, respectively:
In all the above equations, M indicates molecular weight. Lij is phenomenological coefficient and \(Q_{j}^{*}\) is the net heat of transport of component i.
There are several published relations available for estimating net heat of transport. For this study, Eq. (17) has been implemented which is presented by Shukla and Firoozabadi (1998):
where \({\bar {U}_i}\) and \({\bar {V}_i}\) are the partial molar internal energy and the partial molar volume of component i and \({\tau _i}\) is the ratio of the energy of vaporization to the energy of viscous flow. The relation between phenomenological coefficients, Lij, and multi-component diffusion coefficients, \({\mathbf{D}}_{{{\mathbf{ij}}}}^{{\mathbf{M}}}\), is shown in the following equation (Ghorayeb and Firoozabadi 2000a):
where \({f_k}\) is the fugacity of component i that is calculated from Peng–Robinson equation of state. Also, \({\delta _{lk}}\) denotes the Kronecker delta.
Proper boundary conditions are required to solve Eq. (3). As stated earlier, it is considered a porous media bounded by an impervious rock which is resulted to have isolated boundaries with no fluxes:
In other words,
Also, to solve Eq. (2), below boundary conditions are implemented:
Pressure, temperature and composition of a reference point are necessary to have a simultaneous solution of Eqs. (2) and (3) with mentioned boundary conditions. The center point of rectangle is assumed as reference point with \(\left\{ {{y_{0,i}}} \right\}\), P0 and T0 that denote mole fraction of components and reservoir pressure and temperature, respectively.
Among several applicable numerical approaches, decoupled method is used in this study to solve the mentioned set of equations. In this method, first, compositions are assumed to be constant and with solving Eqs. (2) and (3), pressures in each point are estimated by Newton method. After that, with calculated pressures, conservation equation is solved to achieve new compositions. Convergence condition is achieved when absolute relative error of mole fraction between two successive iterations at each point reaches a minimum predefined value such as 10−8. After correction of compositions, pressures and bulk velocities will be corrected in an outer loop. Secant method is implemented for error convergence. Figure 2 illustrates a simple flowchart of described numerical method.
Field data
To cover all objectives stated before, three different sets of data are used. The first aim is to improve compositional gradient prediction by utilizing suitable correlations for calculating molecular diffusion coefficients in gas mixture. For this purpose, a set of data from a gas condensate reservoir reported by Ghorayeb and Firoozabadi (2000) is employed which is shown in Table 1. In part 2, a sample from a supergiant gas condensate reservoir is chosen that is presented in Table 2. This sample is selected among more than 40 PVT samples after passing quality check based on procedure proposed by Osfouri et al. (2014). This set of data is interested because study on the compositional gradient of this supergiant reservoir is a part of comprehensive study on phase behavior of this gas condensate field. For the last part, as compositional gradient is more notable in volatile oil compare with other types of oil such as slightly volatile or black oil a volatile oil sample is selected from data reported by Hamoodi et al. (1996) refer to Table 3.
Results and discussion
Referring to the introduced model and governing equations, initial condition of sample should be single phase to calculate the compositional gradient. So, first, condition of each sample is checked from its two-phase diagram as it is shown in Fig. 3a–c. In Fig. 3a, for gas condensate, sample is in single gas phase with respect to its initial condition. Based on critical point and initial condition on this phase envelope, one can conclude that mentioned gas condensate is in near-critical condition. Figure 3b illustrates that this gas condensate sample no. 2 is also in single phase according to its initial pressure and temperature. Figure 3c represents the condition of volatile oil sample. It is obvious that sample is in single phase and far from its critical condition.
Effect of molecular diffusion coefficients on 2D compositional gradient prediction
Regarding the procedure described in “Mathematical modeling: governing equations and boundary conditions”, compositional gradient in 2D for sample no. 1 (refer to Table 1) is calculated. In the first step, molecular diffusion coefficients are estimated according to Eqs. (7–11), i.e. Sigmund–Bird correlation. Predicted values are listed in Table 4.
Referring to these estimated values and proposed model presented before, with k = 5 md as average value for permeability, 2D gradient of methane (C1) is calculated in a rectangle section of the reservoir with 500 (m) depth and 1500 (m) width. This variation of methane composition is trended in Fig. 4a. Calculations are re-done this time based on Ghorayeb model (Kooijman and Taylor 1991). Values of these parameters are also presented in Table 5 while trend of methane gradient is shown in Fig. 4b.
In both cases, methane is more segregated toward the right-top corner of selected geometry, which is the cold region. Figure 4a, b shows that there is a significant difference in the prediction of methane compositional gradient from these two models. In the proposed model, composition of methane varies about 1 mol% with depth in a specific width (at w = 0 as reference point) and totally it varies more than 4 mol% from right-bottom corner to left-top corner, while in Ghorayeb model, methane gradient with depth is about 0.4 mol% in reference width and is about 0.6 mol% from right-bottom to left-bottom corner. Referring to the values of molecular diffusion coefficients in Tables 4 and 5, it is obvious that calculations from the two mentioned methods are different not only in some magnitudes but also on their signs. It seems that greater magnitude of some molecular diffusion coefficients in model 1 cause to have more molecular diffusivity in multi-component mixture so that segregation occurs better than model 2. Regarding these investigations, it is expected that appropriate correlations could have significant effects on compositional gradient and also distribution of components. To realize this expectation, in the next section, a sample from a gas condensate field is tested by the proposed model and results from model are compared with experimental data.
Effect of natural convection on 2D compositional gradient in gas condensate reservoir
To validate the developed model with experimental data, three test wells from a gas condensate reservoir mentioned in “Field data” are selected. One of them is chosen as target well that its composition is fed to the model while experimental data of two others are used to validate the outputs of model. Figure 5 presents a simple schematic of reservoir location. Well no.1 is target well and Well nos. 2 and 3 are selected for experimental data. Average porosity of 0.03 and average permeability of 15 md are considered for this section of reservoir. Other details are illustrated in schematic figure. Experimental composition of methane is compared with the results from the two earlier mentioned models, i.e. developed model and Ghorayeb model. All three wells have almost same vertical positions with different horizontal locations to cover both forward and backward directions in calculation. It is indicated that for C1 mole% in Well no. 2 (for backward calculations), absolute relative error of results from the developed model is about 0.28% while it is about 0.4% for Ghorayeb model. It is obvious that the proposed model has more reliable results than Ghorayeb model. These investigations are also repeated for Well no. 3 which covers forward prediction of compositional gradient and absolute relative errors are 0.32% and 0.56% for the proposed model and Ghorayeb model, respectively. Good agreement between field data and the results from developed model shows that in addition to having a reliable prediction from this model, the results could use to validate quality and correctness of PVT tests reported for the field of study.
Based on the results obtained from the above validation step, study on gas condensate reservoir was conducted using the developed model. Going back to Eq. 4, it is shown that there are three main mechanisms involved in predicting compositional gradient: molecular diffusion, pressure diffusion and thermal diffusion. In addition, based on the horizontal temperature gradient, natural convection will have an important role. In this section, cross effects of these mechanisms combined with natural convection are studied. Figure 6a, b illustrates the distribution of C1 in selected geometry in two cases of with and without natural convection. It is obvious that in a specific width (width of reference point, w = 0), there is about 7 mol% vertical gradient of C1 in 500 m depth. Also, trend shows that C1 is segregated toward the right-top corner of reservoir. In the next case, shown in Fig. 4b, effect of natural convection is included in calculations by considering k = 10 md. Results illustrate that natural convection is affected not only by the magnitude of vertical gradient, but also by changes in C1 segregation pattern. Natural convection decreases vertical gradient to about 1.5 mol% in comparison with 7 mol% in free convection case. One can conclude that convection causes mixing in flow streams such that a semi-homogenous condition occurs and consequently, species distribute more uniformly. In addition, due to the natural convection of lighter species from region with higher temperature (right side of reservoir) towards region with lower temperature (left side), concentration of C1 will be higher at left-top corner.
In Fig. 7a, b, contour plot of C7+ is presented for both convection-free and natural convection cases. As it is expected, C7+ segregates toward right-bottom corner of geometry under study. In this condition, it varies about 0.76 mol% per 500 m depth. Considering natural convection (k = 10 md) causes to have semi-homogenous conditions due to flow stream mixing. Consequently, vertical gradient decreases to about 0.15 mol%. Also, convection streams displace lighter species to the colder part of reservoir which is yielded to move higher ones to the part with higher temperature.
Results from Fig. 7a, b indicate that trends of changes in compositional gradient are not similar in various magnitude of permeability. In Fig. 8a, b, variations of C1 gradient in vertical and horizontal vs. different values of permeability are illustrated. In convection-free condition, C1 has the maximum gradient in vertical direction. With introducing natural convection effect, vertical gradient reduces sharply, while in contrast, horizontal gradient increases significantly. One can conclude that natural convection brings down the effect of molecular diffusion which resulted to increase horizontal gradient and decrease vertical. With more increase in permeability (stronger convection), more homogeneity in compositions will occur and as a result, horizontal gradient decreases since permeability reaches a value about 15 md; for values greater than 15 md, it is almost constant. On the other hand, after a sharp decrease in vertical gradient with considering natural convection, there is a notable increase in vertical gradient until permeability reaches10 md, after that and with increase in convection effect, vertical gradient decreases while gets constant from k = 30 md. The most probable reason is that with primary increase in convection, streams replace in layers which forces to move species from hot side to the cold side. This replacement at first causes some increase in gradient, but as convection gets stronger, as a result, more homogenous distribution makes reduction in vertical gradient until it reaches almost a constant value.
Effect of natural convection on 2D compositional gradient in volatile oil reservoir
In this part, with implementing Ghorayeb model, 2D compositional gradient in a volatile oil sample (refer to data presented in Table 3) is predicted. Figure 9a, b shows the effect of natural convection on distribution of C1 in a volatile oil sample. As it is illustrated, natural convection displaces C1 segregation from right-top side toward left-top side which is same as the trends resulted from gas condensate. It seems that convection reduces both vertical and horizontal gradients due to uniform distribution of species.
Moreover, Fig. 10a, b shows trends of C7+ mole fraction variation in volatile oil for both convection-free and natural convection cases. C7+ is concentrated in left-bottom side of the reservoir due to its higher molecular weight. In contrast, it is separated towards right side, i.e. the region with more temperature, which is similar to results investigated for C7+ in gas sample.
Figure 11a, b compares gas condensate and volatile oil samples with the same permeability (k = 1 md). It is obviously that trend lines in gas condensate sample are sharper in vertical whereas there are more linear in horizontal direction in volatile oil. Results show that from left-top towards right-bottom corner, methane composition varies about 5.2 mol% in gas condensate sample, whereas this value is about 0.45 mol% in volatile oil sample. Hence, volatile oil sample becomes more uniform in comparison with gas condensate in the same magnitude of permeability. In Fig. 12a, b, which demonstrates variations of density in both gas condensate and volatile oil samples, it is obvious that in gas condensate fluid, density has higher gradient from right-top to left-bottom corner, more than 350 kg/m3 versus 150 kg/m3 in volatile oil; it means that there is more turbulence in gas sample compared with oil sample causing to have higher gradient of C1 in gas condensate. On the other hand, more uniformity in density of volatile oil sample has caused to have almost flat trends of C1 composition. It seems that one logical reason is due to bulk velocity. Figure 13a, b shows contour plot of bulk velocity for two samples. As it is expected, bulk velocity magnitude is higher in gas condensate with respect to volatile oil sample. Magnitude of bulk velocity varies more than 3e−6 m/s from right-top to left-bottom corner in comparison with 3e−7 m/s. Consequently, uniformity in flow streams resulted from lower bulk velocity and higher mixture density in oil sample causes to have almost no significant C1 gradient in volatile oil sample.
Conclusion
Based on a model proposed by Ghorayeb and Firoozabadi (2000) and by implementing Sigmund–Bird correlations for estimating molecular diffusion coefficients in gas mixtures, a thermodynamic model was improved to predict compositional gradient and distribution of methane and plus fraction of reservoir fluids in two-dimensional cross-sectional porous media. From this study, it is investigated that using appropriate correlations for estimating molecular diffusion coefficients remarkably reduces calculation errors. In this work and for a gas condensate sample as a case study, it is concluded that applying the developed model could have more reliable predictions such as reducing relative absolute errors about 50% compared with Ghorayeb model. Also, it is shown that natural convection affects not only the pattern of species distribution, but also it changes compositional gradients in both horizontal and vertical directions significantly. Presence of natural convection could reduce the effect of molecular diffusion, hence vertical gradient decreases. The stronger convection, outcomes from higher permeability, causes more homogenous distribution and consequently decreases compositional gradient. As streams get homogenous, more increase in permeability does not show noticeable variations in results. Effect of natural convection is more noteworthy in gas mixtures because of its lower density and higher bulk velocity which lead to having more turbulency in flow streams followed by more gradient compared with oil mixture. The agreement between results from developed model and experimental data showed that the prediction of model was sufficiently close to reality, such that the model could significantly help reservoir engineers in reservoir managing programs.
Abbreviations
- c :
-
Total molar density
- \(D_{ij}^{M}\) :
-
Molecular diffusion coefficient
- \(D_{i}^{P}\) :
-
Pressure diffusion coefficient
- \(D_{i}^{T}\) :
-
Thermal diffusion coefficient
- \(D_{ij}\) :
-
Mutual diffusion coefficient
- \(f_{i}\) :
-
Fugacity of component i
- g :
-
Gravity acceleration
- h :
-
Reservoir depth
- \(J_{i}\) :
-
Molar diffusive flux of component i
- k :
-
Permeability
- \(k_{Ti}\) :
-
Thermal diffusion ratio of component i
- \(M_{i}\) :
-
Molecular weight of component i
- M :
-
Molecular weight of mixture
- P :
-
Pressure
- [P] :
-
Matrix in Toor correlation
- Pc:
-
Critical pressure
- Q*:
-
Net heat of transport
- R :
-
Universal gas constant
- T :
-
Temperature
- Tc:
-
Critical temperature
- \(\bar{U}_{i}\) :
-
Partial molar internal energy
- V :
-
Velocity vector
- \(\bar{V}_{i}\) :
-
Partial molar volume
- w :
-
Reservoir width
- \(y_{i}\) :
-
Composition of component i
- \(\delta_{ik}\) :
-
Kronecker delta
- \(\rho\) :
-
Total mass density
- \(\rho_{r}\) :
-
Reduced mass density
- \(\tau_{i}\) :
-
Ratio of vaporization energy to viscous energy
- μ :
-
Viscosity
- φ :
-
Porosity
- i,j,k :
-
ith, jth, kth component
- x :
-
Horizontal dimension index
- z :
-
Vertical dimension index
References
Bird RB, Stewart WE, Lightfoot EN (2002) Transport phenomena. Wiley, New York
Da Silva FV, Belery P (1989) Molecular diffusion in naturally fractured reservoirs: a decisive recovery mechanism. In: SPE annual technical conference and exhibition, SPE-19672, 8–11 October 1989, San Antonio, Texas, pp 428–442
Firoozabadi A (1999) Thermodynamic of Hydrocarbon Reservoirs, McGraw-Hill
Firoozabadi A, Dindoruk B (1995) Areal and vertical composional variation in hydrocarbon reservoirs. Reservoir Engineering Research Institute, Palo Alto
Firoozabadi A, Ghorayeb K, Shukla K (2000) Theoretical model of thermal diffusion factors in multicomponent mixtures. AIChE 46(5):892–900
Ghorayeb K, Firoozabadi A (2000a) Molecular, pressure and thermal diffusion in nonideal multicomponent mixtures. AICHE J 46(6):883–891
Ghorayeb K, Firoozabadi A (2000b) Modeling multicomponent diffusion and convection in porous media. SPE J 5(2):158–171
Hamoodi AN, Abed AF, Firoozabadi A (1996) Compositional modeling of two-phase hydrocarbon reservoirs. In: Abu Dhabi international petroleum exhibition and conference, 13–16 October 1996, Abu Dhabi, United Arab Emirates, SPE-36244, pp 756–770
Hasse R (1962) Thermodynamics of irreversible processes, 1st edn. Addison-Wesley Publishing Company, Boston
Hoier L, Whitson CH (2001) Compositional grading: theory and practice. In: SPE reservoir evaluation & engineering, SPE-63085, pp 525–535
Kempers LJTM (2001) A comprehensive thermodynamic theory of the Soret effect in a multicomponent gas, liquid, or solid. J Chem Phys 115(14):6330–6341
Kooijman HA, Taylor R (1991) Estimation of diffusion coefficients in multicomponent liquid systems. Ind Eng Chem Res 30(6):1217–1222
Montel F, Gouel PL (1985) Prediction of compositional grading in a reservoir fluid column. In: SPE annual technical conference and exhibition, 22–26 September 1985, Las Vegas, Nevada, SPE-14410
Nasrabadi H, Ghorayeb A (2006) Two-phase multicomponent diffusion and convection for reservoir initialization. SPE Reserv Eval Eng 9:530–542
Osfouri S, Azin R, Kiani M, Gerami S (2014) A unified approach for quality control of drilled stem test (DST) and PVT data. Gas Process J 2(1):40–50
PVTi Reference Manual (2009) Schlumberger. https://www.slb.com
PVTsim Technical Overview (2016) Calsep. https://www.pvtsimnova.com
Riley MF, Firoozabadi A (1998) Compositional variation in hydrocarbon reservoirs with natural convection and diffusion. AICHE J 44(2):452–464
Shukla K, Firoozabadi A (1998) A new model of thermal diffusion coefficients in binary hydrocarbon mixtures. Ind Eng Chem Res 37:3331–3342
Sigmund PM (1976a) Prediction of molecular diffusion at reservoir conditions. Part II—estimating the effects of molecular diffusion and convective mixing in multi-component systems. J Can Pet 15:53–62
Sigmund PM (1976b) Prediction of molecular diffusion at reservoir conditions. Part I—measurement and prediction of binary dense gas diffusion coefficients. J Can Pet 15:48–57
Toor HL, Seshadri CV, Arnold KR (1965) Diffusion and mass transfer in multicomponent mixtures of ideal gases. AIChE J 11(4):746–757
Whitson CH, Trondheim U, Belery P (1994) Compositional gradients in petroleum reservoirs. In: University of Tulsa centennial petroleum engineering symposium, 29–31 August 1994, Tulsa, Oklahoma, SPE-28000, pp 443–459
WinProp User’s Guide (2013) Computer Modelling Group. https://www.cmgl.ca/winprop
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kiani, M., Osfouri, S., Azin, R. et al. A comprehensive model for the prediction of fluid compositional gradient in two-dimensional porous media. J Petrol Explor Prod Technol 9, 2221–2234 (2019). https://doi.org/10.1007/s13202-019-0624-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-019-0624-y