Abstract
Purpose
Thyroglobulin (Tg) may be released from damaged residual thyroid tissues after radioactive iodine (RAI) therapy in patients with differentiated thyroid carcinoma (DTC). We investigated whether altered levels of serum Tg after recombinant human thyrotropin (rhTSH)-aided RAI therapy could be a prognostic marker in patients with DTC.
Methods
We evaluated 68 patients who underwent RAI therapy after total thyroidectomy. Serum Tg levels were measured just before RAI administration (D0Tg) and 7 days after RAI therapy (D7Tg). Patients with a D0Tg level greater than 2.0 ng/mL were excluded to more precisely evaluate the injury effect of RAI in small remnant tissues. The ratioTg was defined as the D7Tg level divided by that on D0Tg. The therapeutic responses were classified as acceptable or non-acceptable. Finally, we investigated which clinicopathologic parameters were associated with therapeutic response.
Results
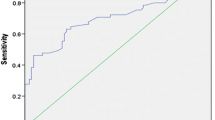
At the follow-up examination, an acceptable response was observed in 50 patients (73.5%). Univariate analysis revealed significant differences in N stage (P = 0.003) and ratioTg (acceptable vs. non-acceptable responses, 21.9 ± 33.6 vs. 3.8 ± 6.5; P = 0.006). In multivariate analysis, only ratioTg significantly predicted an acceptable response (odds ratio 1.104; 95% confidence interval 1.005–1.213; P = 0.040). A ratioTg above 3.5 predicted an acceptable response with a sensitivity of 66.0%, specificity of 83.3%, and accuracy of 70.6% (area under the curve = 0.718; P = 0.006).
Conclusions
Altered levels of serum Tg after RAI therapy, calculated as the ratioTg (D7Tg/D0Tg), significantly predicted an acceptable response in patients with DTC.


Similar content being viewed by others
References
Muratet JP, Giraud P, Daver A, Minier JF, Gamelin E, Larra F. Predicting the efficacy of first iodine-131 treatment in differentiated thyroid carcinoma. J Nucl Med. 1997;38:1362–8.
Kim TY, Kim WB, Kim ES, Ryu JS, Yeo JS, Kim SC, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:1440–5.
Bernier MO, Morel O, Rodien P, Muratet JP, Giraud P, Rohmer V, et al. Prognostic value of an increase in the serum thyroglobulin level at the time of the first ablative radioiodine treatment in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2005;32:1418–21.
Park HJ, Jeong GC, Kwon SY, Min JJ, Bom HS, Park KS, et al. Stimulated serum thyroglobulin level at the time of first dose of radioactive iodine therapy is the most predictive factor for therapeutic failure in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014;48:255–61.
Kim YI, Im HJ, Paeng JC, Cheon GJ, Kang KW, Lee DS, et al. Serum thyroglobulin level after radioiodine therapy (day 3) to predict successful ablation of thyroid remnant in postoperative thyroid cancer. Ann Nucl Med. 2015;29:184–9.
Jeong GC, Song M, Park HJ, Min JJ, Bom HS, Cho SG, et al. Iodine uptake patterns on post-ablation whole body scans are related to elevated serum thyroglobulin levels after radioactive iodine therapy in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2016;50:329–36.
Melo M, Costa G, Ribeiro C, Carrilho F, Martins MJ, da Rocha AG, et al. Stimulated thyroglobulin at recombinant human TSH-aided ablation predicts disease-free status one year later. J Clin Endocrinol Metab. 2013;98:4364–72.
Park HJ, Min JJ, Bom HS, Kim J, Song HC, Kwon SY. Early stimulated thyroglobulin for response prediction after recombinant human thyrotropin-aided radioiodine therapy. Ann Nucl Med. 2017;31:616–22.
Rosario PW, Salles DS, Purisch S. Area under the curve of TSH after levothyroxine withdrawal versus administration of recombinant human TSH (rhTSH): possible implications for tumor growth. Arq Bras Endocrinol Metabol. 2009;53:767–70.
Nishiyama K, Kozuka T, Higashihara T, Miyauchi K, Okagawa K. Acute radiation thyroiditis. Int J Radiat Oncol Biol Phys. 1996;36:1221–4.
Cramp WA, Yatvin MB, Harms-Ringdahl M. Recent developments in the radiobiology of cellular membranes. Acta Oncol. 1994;33:945–52.
Ramakrishnan N, McClain DE, Catravas GN. Membranes as sensitive targets in thymocyte apoptosis. Int J Radiat Biol. 1993;63:693–701.
Bachelot A, Cailleux AF, Klain M, Baudin E, Ricard M, Bellon N, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12:707–11.
Kowalska A, Pałyga I, Gąsior-Perczak D, Walczyk A, Trybek T, Słuszniak A, et al. The cut-off level of recombinant human TSH-stimulated thyroglobulin in the follow-up of patients with differentiated thyroid cancer. PLoS One. 2015;10:e0133852.
Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–9.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2015;26:1–133.
Rivolta CM, Targovnik HM. Molecular advances in thyroglobulin disorders. Clin Chim Acta. 2006;374:8–24.
Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–7.
Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–85.
Funding
This research was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2015M3C1A3056410).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors Minchul Song, Subin Jeon, Sae-Ryung Kang, Zeenat Jabin, Su Woong Yoo, Jung-Joon Min, Hee-Seung Bom, Sang-Geon Cho, Jahae Kim, Ho-Chun Song, and Seong Young Kwon declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
Rights and permissions
About this article
Cite this article
Song, M., Jeon, S., Kang, SR. et al. Response Prediction of Altered Thyroglobulin Levels After Radioactive Iodine Therapy Aided by Recombinant Human Thyrotropin in Patients with Differentiated Thyroid Cancer. Nucl Med Mol Imaging 52, 287–292 (2018). https://doi.org/10.1007/s13139-018-0528-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-018-0528-7