Abstract
Surrogate broodstock technology facilitates the production of donor-derived gametes in surrogates, and comprises transplanting germ cells of a donor into recipients of a different strain or different species. The following applications of this technology are expected in the field of aquaculture: (1) the efficient and reliable production of offspring carrying superior genetic traits by transplanting donor germ cells from a single selected fish with superior traits into many recipient fish; (2) the reduction of the time required to breed fish by using a recipient species with a short generation time to produce gametes of a species with a long generation time; (3) the long-term storage of valuable species or strains as genetic resources by cryopreserving germ cells for transplantation; (4) the mass production of genetically sterile fish by transplanting germ cells of a donor fish that is sterile due to a mutation in the somatic cells into normal recipients without this mutation. It is expected that a combination of these techniques will greatly accelerate the breeding of aquaculture species. It is important to adapt surrogate broodstock technology to a wider range of fishery species and further improve the efficiency of donor-derived gamete production when using surrogate broodstock.
Similar content being viewed by others
Methods to generate surrogate broodstock
Surrogate broodstock technology consists of producing donor-derived gametes in a surrogate fish (recipient individual) by transplanting germ cells of a donor into a recipient of a different strain or species. This technology can be facilitated by the transplantation of a cell suspension from the testis or ovary containing germline stem cells, which will eventually become sperm or eggs, respectively, into larvae immediately after hatching. Even though allogeneic or xenogeneic donor cells are transplanted into recipients, rejection can be avoided because the newly hatched larvae do not have a mature immune system and thus the ability to reject foreign substances (Takeuchi et al. 2004; Okutsu et al. 2007). In addition, donor-derived germline stem cells do not need to be transplanted into the testis or ovary of the recipient larvae. After transplantation into the intraperitoneal cavity by a fine glass pipette, they spontaneously migrate to the immature testis and ovary, into which they are incorporated and initiate spermatogenesis and oogenesis, respectively (Fig. 1) (Takeuchi et al. 2003; Okutsu et al. 2006). Furthermore, it is not necessary to purify the germline stem cells used for transplantation. When testis or ovary tissues are dissociated by proteinase in preparing the cell suspension for transplantation, only germline stem cells migrate to the recipient’s genital ridges for incorporation, while the remaining cells eventually die in the abdominal cavity (Okutsu et al. 2006). Therefore, germline cell transplantation comprises an extremely simple microscopic operation using a stereomicroscope and a coarse motion micromanipulator.
We have successfully generated masu salmon Oncorhynchus masou-producing gametes of rainbow trout Oncorhynchus mykiss using this method (Takeuchi et al. 2004; Okutsu et al. 2007). The remarkable aspect of this technology is that female recipients produce functional eggs derived from donor cells after the transplantation of spermatogonial stem cells prepared from the donor testis (Okutsu et al. 2006) and male recipients produce functional sperm derived from the donor after the transplantation of oogonial stem cells prepared from the donor ovary (Yoshizaki et al. 2010a). Evidently, if the sex of the donor and recipient match, sperm will be generated by spermatogonial stem cells and eggs by oogonial stem cells. Accordingly, donor germline stem cells produce gametes based on the gender of the recipient, not their own gender (Yoshizaki et al. 2010b). When rainbow trout cells were transplanted into a wild-type masu salmon, the masu salmon recipients simultaneously produced gametes of both rainbow trout and masu salmon (Okutsu et al. 2008b). Moreover, it was possible to create a masu salmon that produced only rainbow trout gametes by transplanting germline stem cells derived from diploid rainbow trout into triploid sterile masu salmon (Fig. 2) (Okutsu et al. 2007). As an alternative to triploidization, sterile recipients lacking endogenous germ cells can be generated by gene knockdown or knockout of the dead end (dnd) gene, which is required for the maintenance of primordial germ cells (Saito et al. 2008; Linhartová et al. 2015; Wong and Zohar 2015; Yoshizaki et al. 2016; Wargelius et al. 2016; Li et al. 2017; Octavera and Yoshizaki 2018).
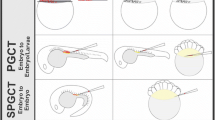
Production of masu salmon surrogate broodstock and next generation of rainbow trout using germline stem cell transplantation. Spermatogonial stem cells from male rainbow trout were transplanted into the peritoneal cavity of newly hatched sterile triploid masu salmon; this resulted in the transplanted trout spermatogonia colonizing the gonads of the recipients, which eventually produced donor-derived eggs or sperm, depending on the sex of the recipients
Similar to dnd knockdown or knockout, the generation of germ cell-less recipients has recently become possible by producing inter-species hybrids in the Sciaenidae. It has been confirmed that these recipients can maintain the ability to produce donor-derived gametes after germline stem cell transplantation (Yoshikawa et al. 2018a). In addition to the method of transplanting germline stem cells into newly hatched larvae, as described above, other methods have been developed. One consists of transplanting migrating primordial germ cells into the blastodisc at the blastula stage before their differentiation into germline stem cells (Saito et al. 2008, 2010). Another method consists of directly transplanting spermatogonial stem cells into an empty gonad after removing the endogenous germ cells of the parent fish by an alkylating agent such as busulfan (Majhi et al. 2009; Lacerda et al. 2010). Details of these methods can be found in other review articles (Lacerda et al. 2013; Yoshizaki and Lee 2018).
By using the above-mentioned intraperitoneal transplantation of germline stem cells, production of the next generation derived from transplanted donor germ cells using surrogate broodstock has been successfully employed in fish producing small eggs, such as yellowtail Seriola quinqueradiata (Morita et al. 2012, 2015), tiger puffer Takifugu rubripes (Hamasaki et al. 2017; Yoshikawa et al. 2018b), nibe croaker Nibea mitsukurii (Yoshikawa et al. 2017), and chub mackerel Scomber japonicus (Tani et al., unpublished data). Accordingly, in principle, it is possible to apply this method to various commercially important fish species, as long as they are oviparous. Thus, when considering the application of this technology, an important question is the extent to which the genetic distance between donor and recipient is acceptable. While further investigation is required to clarify the limits of genetic distance, on the basis of previous research, it is believed that recipients can stably produce both donor-derived eggs and sperm as long as they belong to the same genus (Bar et al. 2016), even if they are of different species (Table 1). Moreover, even if they do not belong to the same genus, at least the production of sperm is usually possible if the donor and recipient belong to the same family (Morita et al. 2015) (Table 1). Recently, we also confirmed the successful production of donor-derived eggs by intergeneric transplantation in species of the Acheilognathinae (Octavera et al., unpublished data) and Salmonidae (Fujihara et al., unpublished data). Examples of the application of surrogate broodstock technology to various aquaculture techniques will be described in the following sections.
Acceleration of breeding by increased mating efficiency
In general, broodstock of marine fish are maintained in a large land-based tank, and fertilized eggs are collected using an egg collection net set at the water outlet of the tank. However, this method is not appropriate for efficient breeding methods that often require the mating of particular parent individuals carrying desirable genetic traits (i.e., selective breeding). In some fish species, males and females with superior traits can be artificially bred using a maturation induction technique using exogenous hormone administration. However, some marine fish are quite sensitive to handling stress, and there is also a risk of losing valuable parent fish during egg or sperm collection. Furthermore, the survival rate is usually low for fertilized eggs obtained by in vitro fertilization compared with naturally spawned eggs. Therefore, for fish species in which in vitro fertilization is difficult, one must rely on the spontaneous oviposition. In group-spawning marine fishes, it is not easy to obtain fertilized eggs from a small number of selected individual fish (in particular with a single male and single female in the tank). In extreme cases, mating between males and females with superior traits may become impossible because they do not reach maturity at the same time.
To overcome these drawbacks, germline stem cells from an individual fish carrying superior traits can be transplanted into a large number of recipient fish to generate a large number of females and males that produce gametes carrying genes associated with the superior traits. Group spawning of the resulting surrogate parent fish thereby leads to fertilization involving eggs and sperm derived from donors with superior traits (Fig. 3) (Morita et al. 2012). As a result, the efficiency of breeding will be much improved. The production of a next generation from surrogate parent fish that received donor-derived germ cells was successful in a group-spawning fish, the chub mackerel, by the administration of a gonadotropin-releasing hormone analogue (Tani et al., unpublished data).
Improvement in breeding efficiency of group spawner using surrogate broodstock. Fish carrying superior traits (black) and recipients (gray) are shown. Germ cell transplantation can produce a large number of recipients from a single donor fish with superior traits. This increases the probability of fish acquiring gametes carrying the donor-derived haplotype. Consequently, group spawning of the resulting recipients with mating between any individual female and male recipient leads to fertilization involving eggs and sperm derived from donors with superior traits
As mentioned above, intra-species transplantation technology can achieve the production of genetically superior offspring from a donor. As in vitro fertilization has not yet been established for large-bodied fish such as the bluefin tuna Thunnus orientalis, intra-species transplantation is expected to be a potentially effective strategy for these. However, inter-species transplantation would have many more advantages. This latter technology is expected to be able to produce bluefin tuna from a small mackerel species such as the chub mackerel. Adult bluefin tuna are quite large and require 3–5 years to reach sexual maturity. In contrast, adult chub mackerel, which also belongs to the Scombridae, weigh 300 g and reach maturity in 1 year. Therefore, if the chub mackerel is able to produce eggs and sperm of bluefin tuna, the space, labor, and cost required for maintenance of the broodstock would be minimized. In addition, transplanting germline stem cells from bluefin tuna with superior traits into a small fish of the Scombridae would create genetically improved seedlings of bluefin tuna, which would be a far more efficient method than using bluefin tuna itself as a surrogate broodstock.
Acceleration of breeding by reducing the generation period
One of the major obstacles to breeding fish is the long generation time of many valuable aquacultural fish species. Mating experiments are indispensable in selective breeding programs, and there is strong demand to reduce the generation time for breeding species for the aquaculture industry and fish research. As shown by the example above, which describes the production of bluefin tuna from a small mackerel, it is possible to significantly shorten the period necessary for breeding using a fish species with a short generation time as surrogate broodstock. We have successfully produced eggs and sperm of tiger puffer more quickly by transplanting germline stem cells of this species into grass puffer Takifugu niphobles (Hamasaki et al. 2017). Generally, male tiger puffers require 2 years to mature, whereas female tiger puffers require 3 years to mature. Reportedly, both sexes of the grass puffer can mature within a year when the water temperature is controlled and photoperiod manipulated (Yoshiura et al., personal communication). Similarly, it is now possible to produce eggs and sperm of Chinook salmon Oncorhynchus tshawytscha (which normally require 3–5 years to mature) in 1 and 2 years, respectively, when using rainbow trout as surrogate broodstock (Namura et al., unpublished data).
The time required for breeding can be substantially reduced by the selection of individual fish with superior traits during the juvenile stages and by transplanting germline stem cells of the selected fish into a fish species with a short generation time. In recent years, selection using DNA markers has enabled faster and easier identification of target phenotypes based on the DNA analysis of larvae (Abdelrahman et al. 2017). Once the target juveniles or other young have been identified, their germline stem cells can be isolated and transplanted into a surrogate broodstock with a shorter generation time. Therefore, the establishment of a new, superior breed is then be possible in a much shorter time by repeating the above procedure for several generations. Thus, the combination of genome-based selection and surrogate broodstock technology may be a breakthrough for the future of fish breeding.
Cryopreservation of germline stem cells from a superior breed
Cryopreservation has not yet been fully adapted for fish because fish eggs are relatively large and rich in lipids and egg yolk (Mazur et al. 2008). Therefore, there is no technology available for permanently preserving the precious genetic resources of fish breeds, even though some breeds have been successfully developed over many years using a variety of breeding methods. In fact, although many clones and strains with unique genetic characteristics were created in various research stations of prefectural fisheries during the 1980s using various manipulation methods for chromosome sets, many fish breeds have already been lost due to the difficulty of maintaining these clones and strains.
Cryopreservation of germline stem cells can be easily performed in liquid nitrogen because the cells are small (approximately 10 µm) and do not contain much lipid or egg yolk. A method of freezing the testis of immature individuals (containing a large number of immature spermatogonia) in liquid nitrogen is well established, making it theoretically possible to permanently store germline stem cells within the testis in a frozen state (Yoshizaki et al. 2011; Yoshizaki and Lee 2018). In fact, our group showed that there was no decline in survival rate even after we thawed the testis of rainbow trout 5 years after freezing them in liquid nitrogen (Lee et al. 2013, 2016a). Also, it has already been shown that gametes develop normally in the gonads of surrogate fish and that sperm and eggs can be produced from frozen cells even when the cryopreserved cells are thawed and transplanted into recipient fish (Fig. 4) (Lee et al. 2013, 2015). Similarly, it is now possible to cryopreserve ovaries and to isolate and transplant germline stem cells derived from them (Lee et al. 2016b). Cryopreservation is a powerful method for preserving precious genetic resources as this technology requires no special and expensive equipment and is feasible as long as liquid nitrogen and cryo-containers are available. Because a cryopreservation technique for fish eggs is not yet available, a combination of cryopreservation and transplantation of germline stem cells could be extremely important for preserving the genetic resources of endangered species [details of these techniques can be found in other review articles (Yoshizaki et al. 2011; Yoshizaki and Lee 2018; de Siqueira-Silva et al. 2018)].
Long-term preservation of superior fish breeds by freezing germline stem cells. Fish carrying superior traits (black) and recipients (gray) are shown. Germline stem cells can be maintained semi-permanently in liquid nitrogen. Frozen germline stem cells are transplanted into recipients to obtain functional gametes. By mating male and female recipients, fish breeds carrying superior traits can be regenerated by using frozen genetic material
Producing sterile fish
After creating a superior fish breed via selective breeding, there is a risk that the next generation (seedlings with excellent traits) could be mass-produced, even unintentionally, by a third party by breeding first-generation seedlings and raising them to sexual maturity. The resulting seedlings could be sold as “pirated” versions of the original seedlings created with a great deal of effort over a long period of time. This would make it difficult for breeders to profit from their work. To solve this problem, it will be necessary to develop measures for sterilizing seed before selling it. Triploids created from chromosome manipulation would be major candidates for this (Piferrer et al. 2009); however, triploidization leads to a reduction in survival rates, and the triploid rate itself is not very high in some fish species (especially in marine species).
Gene knockdown techniques that specifically suppress the translation of a target gene and gene knockout techniques that disrupt target gene sequences have been recently developed in fishes (Huang et al. 2012; Zhu and Ge 2018). It is possible to create sterile fish by inhibiting the functioning of dnd genes, which are necessary to maintain primordial germ cells (described above as a way to generate surrogate broodstock). However, the gene knockdown and knockout techniques require individual microinjection of the antisense oligonucleotide and guide RNA, together with Cas9 protein, into fertilized eggs under a microscope. This, however, is not a practical method because gametes cannot be obtained from sterile individuals produced by these methods and microinjection into the fertilized egg is required every time to produce sterile fish.
Nagasawa et al. (2018) developed a method to mass produce sterile individuals using surrogate broodstock. Oogenesis of fish generally progresses when ovaries receive stimulation from follicle-stimulating hormone (FSH) secreted from the pituitary gland (Clelland and Peng 2009). The receptor for FSH is expressed not in germ cells but in supporting cells of the ovaries surrounding the germ cells. Female Japanese medaka with a homozygous mutation in the FSH receptor gene are sterile (Murozumi et al. 2014). However, male mutant medaka mature normally (Murozumi et al. 2014). Thus, we isolated germ cells from mutant (sterile) medaka and transplanted them into surrogate parent medaka that carried a wild-type FSH receptor. Because the receptor gene is dispensable for the germ cell itself, transplanted germ cells in the ovary of surrogate parent medaka are nursed by the ovarian supporting cells of the recipient and eventually produce normal eggs. When we fertilized these eggs with sperm of a pseudo-male of a FSH-receptor-mutant medaka (masculinized XX genotype medaka), all of the next generation was XX (and all-sterile females). Thus, it was possible to mass produce sterile individuals with disrupted FSH receptor genes (Nagasawa et al. 2018). In principle, this method can be applied to a wide variety of aquaculture fish species. If we could identify a gene that makes both males and females sterile via gene knockout, it would be possible to mass produce sterile fish by only mating males and females of the surrogate broodstock that carry the knocked-out gene (Fig. 5).
Mass production of genetically sterile fish by surrogate broodstock technology. Donor (black) and recipients (gray) are shown. Although mutations in supporting cells cause sterility in donor fish, these fish carry functional germ cells. If germ cells from the mutant (sterile) donor are transplanted into wild type recipients carrying functional supporting cells, the transplanted germ cells are nursed by the supporting cells in the gonad of the recipient and eventually produce gametes carrying the mutation
The above-described approach for disrupting the somatic genes related to gonadal maturation (via gene editing) is useful for preventing not only the property loss of superior breeds but also escaped fish from fish farms from breeding in the wild. Salmon escape is of concern because the escaped fish return to rivers to breed with wild populations, which disrupts the genetic composition of wild stocks (Glover et al. 2013). Indeed, the escape of Atlantic salmon has become an environmental problem in Norway and elsewhere. The mass production of genetically sterile individuals can reduce the negative impacts of aquaculture on natural ecosystems. The commercialization of genetically modified salmon began in Canada in 2017 (Waltz 2017), and there has been research on applying genome-editing technology to the breeding of farm-raised animals (including fish). We think that the sterilization technique described here could be extremely effective in preventing genetically modified and genome-edited individuals from crossbreeding with wild fish when they escape from fish farms.
Conclusion
In summary, surrogate broodstock technology can be useful to accelerate breeding and preserve the genetic information of broodstock. It can also be used to prevent the unauthorized production of pirated fish seedlings. Surrogate broodstock technology does not artificially manipulate cellular contents (unlike gene recombination or nuclear transfer technologies), which could make it advantageous for application on an industrial scale. However, several problems still have to be resolved before surrogate broodstock technology can be applied to the aquaculture of fish. Some fish species possess low concentrations of germline stem cells in their genital glands. In such species, the use of maturing young fish or immature adult fish (rather than fully mature adult fish) will allow the easy and efficient transplantation of germ cells because the concentration of germline stem cells is relatively high in the gonads of these fish. However, if younger fish are not available and one must use mature adults carrying large numbers of developed germ cells, germline stem cells must be enriched prior to transplantation. Several methods for isolating cell populations containing high concentrations of transplantable germline stem cells from mature testis have recently been developed by using a cell sorter (Kise et al. 2012; Hayashi et al. 2014; Ichida et al. 2017). Further, monoclonal antibodies that specifically recognize spermatogonia containing a high concentration of germline stem cells have been recently generated (Hayashi et al., unpublished data). The use of such antibodies concentrates transplantable cells via magnetic activated cell sorting without the need for any expensive equipment, including a cell sorter.
Another potential problem with transplanting germ cells is that the efficiency of transplantation can vary with the season, e.g., recent research on adult rainbow trout showed that clear seasonality exists in the amount of transplantable spermatogonia produced by their gonads (Sato et al. 2017). Hence, to maximize germ cell transplantation efficiency, it is important to take into account the time of year when germline stem cells accumulate in the gonads of adult donor fish.
A number of the technologies discussed above have recently become useful for both freshwater and marine fishes. We are hopeful that these technologies will be applied to the breeding of a wider range of aquaculture species in the near future.
References
Abdelrahman H, ElHady M, Alcivar-Warren A, Allen S, Al-Tobasei R, Bao L, Beck B, Blackburn H, Bosworth B, Buchanan J, Chappell J, Daniels W, Dong S, Dunham R, Durland E, Elaswad A, Gomez-Chiarri M, Gosh K, Guo X, Hackett P et al (2017) Aquaculture genomics, genetics and breeding in the United States: current status, challenges, and priorities for future research. BMC Genom 18:191
Bar I, Smith A, Bubner E, Yoshizaki G, Takeuchi Y, Yazawa R, Chen BN, Cummins S, Elizur A (2016) Assessment of yellowtail kingfish (Seriola lalandi) as a surrogate host for the production of southern bluefin tuna (Thunnus maccoyii) seed via spermatogonial germ cell transplantation. Reprod Fertil Dev 28:2051–2064
Clelland E, Peng C (2009) Endocrine/paracrine control of zebrafish ovarian development. Mol Cell End 312:42–52
de Siqueira-Silva DH, Saito T, Dos Santos-Silva AP, da Silva Costa R, Psenicka M, Yasui GS (2018) Biotechnology applied to fish reproduction: tools for conservation. Fish Physiol Biochem 44:1469–1485
Glover KA, Pertoldi C, Besnier F, Wennevik V, Kent M, Skaala O (2013) Atlantic salmon populations invaded by farmed escapees: quantifying genetic introgression with a Bayesian approach and SNPs. BMC Genet 14:74
Hamasaki M, Takeuchi Y, Yazawa R, Yoshikawa S, Kadomura K, Yamada T, Miyaki K, Kikuchi K, Yoshizaki G (2017) Production of tiger puffer Takifugu rubripes offspring from triploid grass puffer Takifugu niphobles parents. Mar Biotechnol 19:579–591
Hayashi M, Sato M, Nagasaka Y, Sadaie S, Kobayashi S, Yoshizaki G (2014) Enrichment of spermatogonial stem cells using side population in teleost. Biol Reprod 91:23
Higuchi K, Takeuchi Y, Miwa M, Yamamoto Y, Tsunemoto K, Yoshizaki G (2011) Colonization, proliferation, and survival of intraperitoneally transplanted yellowtail Seriola quinqueradiata spermatogonia in nibe croaker Nibea mitsukurii recipient. Fish Sci 77:69–77
Huang P, Zhu Z, Lin S, Zhang B (2012) Reverse genetic approaches in zebrafish. J Genet Genom 39:421–433
Ichida K, Kise K, Morita T, Yazawa R, Takeuchi Y, Yoshizaki G (2017) Flow-cytometric enrichment of Pacific bluefin tuna type A spermatogonia based on light-scattering properties. Theriogenology 101:91–98
Kise K, Yoshikawa H, Sato M, Tashiro M, Yazawa R, Nagasaka Y, Takeuchi Y, Yoshizaki G (2012) Flow-cytometric isolation and enrichment of teleost type A spermatogonia based on light-scattering properties. Biol Reprod 86:107
Lacerda SM, Batlouni SR, Costa GM, Segatelli TM, Quirino BR, Queiroz BM, Kalapothakis E, Franca LR (2010) A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PLoS One 5:e10740
Lacerda SM, Costa GM, Campos-Junior PH, Segatelli TM, Yazawa R, Takeuchi Y, Morita T, Yoshizaki G, França LR (2013) Germ cell transplantation as a potential biotechnological approach to fish reproduction. Fish Physiol Biochem 39:3–11
Lee S, Iwasaki Y, Shikina S, Yoshizaki G (2013) Generation of functional eggs and sperm from cryopreserved whole testes. Proc Natl Acad Sci USA 110:1640–1645
Lee S, Seki S, Katayama N, Yoshizaki G (2015) Production of viable trout offspring derived from frozen whole fish. Sci Rep 5:16045
Lee S, Iwasaki Y, Yoshizaki G (2016a) Long-term (5 years) cryopreserved spermatogonia have high capacity to generate functional gametes via interspecies transplantation in salmonids. Cryobiology 73:286–290
Lee S, Katayama N, Yoshizaki G (2016b) Generation of juvenile rainbow trout derived from cryopreserved whole ovaries by intraperitoneal transplantation of ovarian germ cells. Biochem Biophys Res Commun 478:1478–1483
Li Q, Fujii W, Naito K, Yoshizaki G (2017) Application of dead end-knockout zebrafish as recipients of germ cell transplantation. Mol Reprod Dev 84:1100–1111
Linhartová Z, Saito T, Kaspar V, Rodina M, Praskova E, Hagihara S, Psenicka M (2015) Sterilization of sterlet Acipenser ruthenus by using knockdown agent, antisense morpholino oligonucleotide, against dead end gene. Theriogenology 84:1246–1255.e1
Lujić J, Marinović Z, Bajec SS, Djurdjevič I, Urbányi B, Horváth Á (2018) Interspecific germ cell transplantation: a new light in the conservation of valuable Balkan trout genetic resources? Fish Physiol Biochem 44:1487–1498
Majhi SK, Hattori RS, Yokota M, Watanabe S, Struessmann CA (2009) Germ cell transplantation using sexually competent fish: an approach for rapid propagation of endangered and valuable germlines. PLoS One 4:e6132
Mazur P, Leibo SP, Seidel GE Jr (2008) Cryopreservation of the germplasm of animals used in biological and medical research: importance, impact, status, and future directions. Biol Reprod 78:2–12
Morita T, Kumakura N, Morishima K, Mitsuboshi T, Ishida M, Hara T, Kudo S, Miwa M, Ihara S, Higuchi K, Takeuchi Y, Yoshizaki G (2012) Production of donor-derived offspring by allogeneic transplantation of spermatogonia in the yellowtail (Seriola quinqueradiata). Biol Reprod 86:176
Morita T, Morishima K, Miwa M, Kumakura N, Kudo S, Ichida K, Mitsuboshi T, Takeuchi Y, Yoshizaki G (2015) Functional sperm of the yellowtail (Seriola quinqueradiata) were produced in the small-bodied surrogate, jack mackerel (Trachurus japonicus). Mar Biotechnol 17:644–654
Murozumi N, Nakashima R, Hirai T, Kamei Y, Ishikawa-Fujiwara T, Todo T, Kitano T (2014) Loss of follicle-stimulating hormone receptor function causes masculinization and suppression of ovarian development in genetically female medaka. Endocrinology 155:3136–3145
Nagasawa K, Ishida M, Octavera A, Kusano K, Kezuka F, Kitano T, Yoshiura Y, Yoshizaki G (2018) Novel method for mass producing genetically sterile fish from surrogate broodstock via spermatogonial transplantation. Biol Reprod. https://doi.org/10.1093/biolre/ioy204 (Epub ahead of print)
Octavera A, Yoshizaki G (2018) Production of donor-derived offspring by allogeneic transplantation of spermatogonia in Chinese rosy bitterling. Biol Reprod. https://doi.org/10.1093/biolre/ioy236 (Epub ahead of print)
Okutsu T, Suzuki K, Takeuchi Y, Takeuchi T, Yoshizaki G (2006) Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci USA 103:2725–2729
Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G (2007) Production of trout offspring from triploid salmon parents. Science 317:1517
Okutsu T, Kobayashi T, Takeuchi Y, Yoshizaki G (2008a) Identification of donor-derived germ cells and spermatozoa in xenogeneic recipient using species-specific primers against vasa gene in germ cell transplantation experiments. Fish Genet Breed Sci 37:29–36
Okutsu T, Takeuchi Y, Yoshizaki G (2008b) Spermatogonial transplantation in fish: production of trout offspring from salmon parents. In: Tsukamoto K et al (eds) Fisheries for global welfare and environment. TERRAPUB, Tokyo, pp 209–219
Pacchiarini T, Sarasquete C, Cabrita E (2014) Development of interspecies testicular germ-cell transplantation in flatfish. Reprod Fertil Dev 26:690–702
Piferrer F, Beaumont A, Falguiere JC, Flajshans M, Haffray P, Colombo L (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293:125–156
Pšenička M, Saito T, Linhartová Z, Gazo I (2015) Isolation and transplantation of sturgeon early-stage germ cells. Theriogenology 83:1085–1092
Saito T, Goto-Kazeto R, Arai K, Yamaha E (2008) Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol Reprod 78:159–166
Saito T, Goto-Kazeto R, Fujimoto T, Kawakami Y, Arai K, Yamaha E (2010) Inter-species transplantation and migration of primordial germ cells in cyprinid fish. Intl J Dev Biol 54:1481–1486
Sato M, Hayashi M, Yoshizaki G (2017) Stem cell activity of type A spermatogonia is seasonally regulated in rainbow trout. Biol Reprod 96:1303–1316
Takeuchi Y, Yoshizaki G, Takeuchi T (2003) Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout. Biol Reprod 69:1142–1149
Takeuchi Y, Yoshizaki G, Takeuchi T (2004) Biotechnology: surrogate broodstock produces salmonids. Nature 430:629–630
Waltz E (2017) First genetically engineered salmon sold in Canada. Nature 548:148
Wargelius A, Leininger S, Skaftnesmo KO, Kleppe L, Andersson E, Taranger GL, Schulz RW, Edvardsen RB (2016) Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci Rep 6:21284
Wong TT, Zohar Y (2015) Production of reproductively sterile fish by a non-transgenic gene silencing technology. Sci Rep 5:15822
Yazawa R, Takeuchi Y, Higuchi K, Yatabe T, Kabeya N, Yoshizaki G (2010) Chub mackerel gonads support colonization, survival, and proliferation of intraperitoneally transplanted xenogenic germ cells. Biol Reprod 82:896–904
Yazawa R, Takeuchi Y, Morita T, Ishida M, Yoshizaki G (2013) The Pacific bluefin tuna (Thunnus orientalis) dead end gene is suitable as a specific molecular marker of type A spermatogonia. Mol Reprod Dev 80:871–880
Yoshikawa H, Takeuchi Y, Ino Y, Wang J, Iwata G, Kabeya K, Yazawa R, Yoshizaki G (2017) Efficient production of donor-derived gametes from triploid recipients following intra-peritoneal germ cell transplantation into a marine teleost, Nibe croaker (Nibea mitsukurii). Aquaculture 478:35–47
Yoshikawa H, Ino Y, Shigenaga K, Katayama T, Kuroyanagi M, Yoshiura Y (2018a) Production of tiger puffer Takifugu rubripes from cryopreserved testicular germ cells using surrogate broodstock technology. Aquaculture 493:302–313
Yoshikawa H, Xu D, Ino Y, Yoshino T, Hayashida T, Wang J, Yazawa R, Yoshizaki G, Takeuchi Y (2018b) Hybrid sterility in fish caused by mitotic arrest of primordial germ cells. Genetics 209:507–521
Yoshizaki G, Lee S (2018) Production of live fish derived from frozen germ cells via germ cell transplantation. Stem Cell Res 29:103–110
Yoshizaki G, Ichikawa M, Hayashi M, Iwasaki Y, Miwa M, Shikina S, Okutsu T (2010a) Sexual plasticity of ovarian germ cells in rainbow trout. Development 137:1227–1230
Yoshizaki G, Okutsu T, Ichikawa M, Hayashi M, Takeuchi Y (2010b) Sexual plasticity of rainbow trout germ cells. Anim Reprod 7:187–196
Yoshizaki G, Fujinuma K, Iwasaki Y, Okutsu T, Shikina S, Yazawa R, Takeuchi Y (2011) Spermatogonial transplantation in fish: a novel method for the preservation of genetic resources. Comp Biochem Physiol Part D Genom Proteom 6:55–61
Yoshizaki G, Takashiba K, Shimamori S, Fujinuma K, Shikina S, Okutsu T, Kume S, Hayashi M (2016) Production of germ cell-deficient salmonids by dead end gene knockdown, and their use as recipients for germ cell transplantation. Mol Reprod Dev 83:298–311
Zhu B, Ge W (2018) Genome editing in fishes and their applications. Gen Comp Endocrinol 257:3–12
Acknowledgements
We thank Dr. J. A. Luckenbach (Northwest Fisheries Science Center, National Oceanic and Atmospheric Administration, USA) for reviewing the manuscript. This work was partly supported by a Grant-in-Aid for Scientific Research (KAKENHI) on Innovative Areas, Establishment of Gamete Integrity (18H05545), Research and Development Program for Future Creation (18077249) of the Japan Science and Technology Agency, and by the Ocean Resource Use Promotion Technology Development Program of the Ministry of Education, Culture, Sports, Science and Technology (to G. Y.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published with support by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant no. JP 262003.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yoshizaki, G., Yazawa, R. Application of surrogate broodstock technology in aquaculture. Fish Sci 85, 429–437 (2019). https://doi.org/10.1007/s12562-019-01299-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01299-y