Abstract
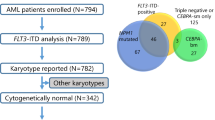
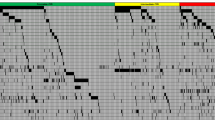
Clinical outcomes and the genetic background of acute myeloid leukemia (AML) in adolescent and young adults (AYAs) are known to differ in younger children and older adults. To clarify the impact of genetic mutations on clinical outcomes of AYAs with AML, we analyzed data from the JPLSG AML-05 and JALSG AML201 studies. AYAs aged 15–39 years (n = 103) were included. FLT3-ITD, KIT, CEBPA, NRAS, KRAS, WT1, MLL-PTD, and NPM1 mutations were analyzed. Overall survival (OS) of the AYAs was 61% and event-free survival was 38% at 3 years. FLT3-ITD (HR 2.10; 95% CI 1.07–4.12; p = 0.031) and NPM1 (HR 0.24; 95% CI 0.06–1.00; p = 0.050) mutations were associated with risk of overall mortality in multivariate analysis. OS was significantly different according to FLT3-ITD and NPM1 mutation status (p = 0.03). Survival was 100% with NPM1 mutations in the absence of FLT3-ITD and 35% (95% CI 14–57%) with FLT3-ITD in the absence of NPM1 mutations. The OS of AYAs, children (n = 413) and older adults (n = 124) of the AML-05 and AML201 participants were significantly different (p < 0.0001). This is the first report to combine clinical and genetic data of AYA AML from the major Japanese pediatric and adult study groups.



Similar content being viewed by others
References
Creutzig U, Buchner T, Sauerland MC, Zimmermann M, Reinhardt D, Dohner H, et al. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer. 2008;112:562–71.
Rubnitz JE, Pounds S, Cao X, Jenkins L, Dahl G, Bowman WP, et al. Treatment outcome in older patients with childhood acute myeloid leukemia. Cancer. 2012;118:6253–9.
Canner J, Alonzo TA, Franklin J, Freyer DR, Gamis A, Gerbing RB, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children’s Oncology Group. Cancer. 2013;119:4162–9.
Tomizawa D, Watanabe T, Hanada R, Horibe K, Horikoshi Y, Iwamoto S, et al. Outcome of adolescent patients with acute myeloid leukemia treated with pediatric protocols. Int J Hematol. 2015;102:318–26.
Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood. 2006;107:4011–20.
Brown P, McIntyre E, Rau R, Meshinchi S, Lacayo N, Dahl G, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007;110:979–85.
Hollink IH, Zwaan CM, Zimmermann M, Arentsen-Peters TC, Pieters R, Cloos J, et al. Favorable prognostic impact of NPM1 gene mutations in childhood acute myeloid leukemia, with emphasis on cytogenetically normal AML. Leukemia. 2009;23:262–70.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89.
Balgobind BV, Hollink IH, Arentsen-Peters ST, Zimmermann M, Harbott J, Beverloo HB, et al. Integrative analysis of type-I and type-II aberrations underscores the genetic heterogeneity of pediatric acute myeloid leukemia. Haematologica. 2011;96:1478–87.
Kihara R, Nagata Y, Kiyoi H, Kato T, Yamamoto E, Suzuki K, et al. Comprehensive analysis of genetic alterations and their prognostic impacts in adult acute myeloid leukemia patients. Leukemia. 2014;28:1586–95.
Tokumasu M, Murata C, Shimada A, Ohki K, Hayashi Y, Saito AM, et al. Adverse prognostic impact of KIT mutations in childhood CBF-AML: the results of the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05 trial. Leukemia. 2015;29:2438–41.
Matsuo H, Nakamura N, Tomizawa D, Saito AM, Kiyokawa N, Horibe K, et al. CXCR4 overexpression is a poor prognostic factor in pediatric acute myeloid leukemia with low risk: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Pediatr Blood Cancer. 2016;63:1394–9.
Matsuo H, Kajihara M, Tomizawa D, Watanabe T, Saito AM, Fujimoto J, et al. Prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Blood Cancer J. 2014;4:e226.
Shiba N, Ohki K, Kobayashi T, Hara Y, Yamato G, Tanoshima R, et al. High PRDM16 expression identifies a prognostic subgroup of pediatric acute myeloid leukaemia correlated to FLT3-ITD, KMT2A-PTD, and NUP98-NSD1: the results of the Japanese Paediatric Leukaemia/Lymphoma Study Group AML-05 trial. Br J Haematol. 2016;172:581–91.
Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood. 2011;117:2358–65.
Miyawaki S, Ohtake S, Fujisawa S, Kiyoi H, Shinagawa K, Usui N, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood. 2011;117:2366–72.
Tomizawa D, Tawa A, Watanabe T, Saito AM, Kudo K, Taga T, et al. Appropriate dose reduction in induction therapy is essential for the treatment of infants with acute myeloid leukemia: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol. 2013;98:578–88.
Cox DR. Regression models and life-tables. J R Stat Soc. 1972;B34:187–200.
Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. Second ed. New York: Springer; 2003.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65.
Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–8.
Pemmaraju N, Kantarjian H, Ravandi F, Nogueras-Gonzalez GM, Huang X, O’Brien S, et al. Patient characteristics and outcomes in adolescents and young adults (AYA) with acute myeloid leukemia (AML). Clin Lymphoma Myeloma Leuk. 2016;16(213–22):e2.
Woods WG, Franklin AR, Alonzo TA, Gerbing RB, Donohue KA, Othus M, et al. Outcome of adolescents and young adults with acute myeloid leukemia treated on COG trials compared to CALGB and SWOG trials. Cancer. 2013;119:4170–9.
Wennstrom L, Edslev PW, Abrahamsson J, Norgaard JM, Floisand Y, Forestier E, et al. Acute myeloid leukemia in adolescents and young adults treated in pediatric and adult departments in the nordic countries. Pediatr Blood Cancer. 2016;63:83–92.
Webb DK, Harrison G, Stevens RF, Gibson BG, Hann IM, Wheatley K, et al. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia. Blood. 2001;98:1714–20.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74.
Majhail NS, Brazauskas R, Hassebroek A, Bredeson CN, Hahn T, Hale GA, et al. Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2012;18:861–73.
Burke MJ, Gossai N, Cao Q, Macmillan ML, Warlick E, Verneris MR. Similar outcomes between adolescent/young adults and children with AML following allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2014;49:174–8.
Tomizawa D, Tanaka S, Kondo T, Hashii Y, Arai Y, Kudo K, et al. Allogeneic hematopoietic stem cell transplantation for adolescents and young adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2017;23:1515–22.
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45.
Acknowledgements
The authors thank all the investigators and members of participating hospitals in AML studies conducted by the JALSG and the JPLSG. This work was supported in part by a grant for Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED), and by the National Cancer Center Research and Development Fund (26-A-24).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Miyachi reports grant from AMED and personal fees for consulting from BML. Inc. Dr. Saito reports grants from Ministry of Health, Labor and Welfare, grants from AMED, during the conduct of the study. Dr. Asou reports grants from Nippon Shinyaku Co., Ltd., grants and personal fees from Kyowa Hakko Kirin Co., Ltd., grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants from Astellas Pharma Inc., grants and personal fees from Sumitomo Dainippon Pharma Co., Ltd., grants from Toyama Chemical Co., Ltd., grants and personal fees from Asahi Kasei Pharma Co., Ltd., personal fees from Boehringer Ingelheim Japan Inc., personal fees from Bristol-Myers Squibb Co., Ltd., personal fees from Nippon Kayaku Co., Ltd., personal fees from Yakult Honsha Co., Ltd., outside the submitted work. Dr. Miyazaki reports grants from AMED, grants from National Cancer Center Research and Development Fund, during the conduct of the study; grants and personal fees from Chugai Pharma, grants and personal fees from Kyowa Hakko Kirin Co., Ltd., grants and personal fees from Astellas Pharma, personal fees from Dainippon Sumitomo, personal fees from Celgene Japan, grants and personal fees from Novartis Japan, outside the submitted work. Dr. Usui reports grants from AMED, during the conduct of the study; personal fees from Astellas Pharma. Inc., grants and personal fees from Bristol-Myers Squibb Co., Ltd., grants and personal fees from Celgene Co., Ltd., personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from CIMIC Co., Ltd., personal fees from Eli Lilly Japan, grants and personal fees from Fujimoto Pharmaceutical Co., Ltd., personal fees from Huya Bioscience International, personal fees from Janssen Pharmaceutical Co., Ltd., personal fees from Kyowa Hakko Kirin Co., Ltd., personal fees from Nippon Boehringer-Ingelheim Co., Ltd., grants from Nippon Shinyaku Pharmaceutical Co., Ltd., grants from Novartis Pharma, grants and personal fees from Otsuka Pharmaceutical Co., Ltd., grants and personal fees from Pfizer Co., Ltd., personal fees from SymBio Pharmaceuticals Co., Ltd., grants and personal fees from Sysmex Co., Ltd., personal fees from Takeda Bio Development Center Ltd., personal fees from Zenyaku Kogyo Co., Ltd., outside the submitted work. Dr. Kobayashi reports grants from Kyowa Kirin, grants from Shionogi, grants from Zenyaku Kogyo, grants from Taiho Pharma, grants from Chugai Pharmaceutical, grants from Asahi Kasei Pharma, grants from Astellas, grants from Eisai, grants from Ono Pharmaceutical, grants from Toyama Chemical, grants from Takeda Pharmaceutical, grants from MSD, outside the submitted work. Dr. Ogawa reports grants and personal fees from KAN Research Institute, Inc., other from Asahi Genomics Co., Ltd., grants from Nippon Shinyaku Co., Ltd., grants from Takeda Pharmaceutical Co., Ltd., outside the submitted work. Dr. Naoe reports grants from Fujifilm Corporation, outside the submitted work; In addition, Dr. Naoe has a patent null pending. Dr. Kiyoi reports grants from AMED, grants from National Cancer Center Research and Development Fund, during the conduct of the study; grants from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Bristol-Myers Squibb, grants from Kyowa Hakko Kirin Co., Ltd., grants from Zenyaku Kogyo Co., Ltd., grants from FUJIFILM Corporation, grants from Nippon Boehringer Ingelheim Co., Ltd., grants and personal fees from Astellas Pharma Inc., grants from Celgene Corporation, personal fees from Daiichi Sankyo Co., Ltd., grants and personal fees from Pfizer Japan Inc, grants from Nippon Shinyaku Co., Ltd., grants from Eisai Co., Ltd., grants from Takeda Pharmaceutical Co., Ltd., grants from Ono Pharmaceutical Co., Ltd., grants from Japan Blood Products Organization, outside the submitted work. The other authors declare no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kuwatsuka, Y., Tomizawa, D., Kihara, R. et al. Prognostic value of genetic mutations in adolescent and young adults with acute myeloid leukemia. Int J Hematol 107, 201–210 (2018). https://doi.org/10.1007/s12185-017-2340-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2340-z