Abstract
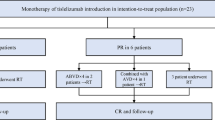
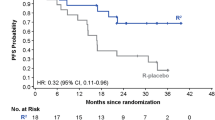
Recent large-scale randomized clinical trials in Europe and the US demonstrated that maintenance therapy with rituximab significantly improved the progression-free survival (PFS) in indolent B-cell non-Hodgkin lymphoma (B-NHL) patients, especially those with follicular lymphoma (FL). However, rituximab maintenance has not been approved in Japan, because there are no clinical data supporting the benefit of rituximab maintenance in Japanese patients. Therefore, we conducted a single-arm, multicenter bridging study in previously untreated indolent B-NHL patients with high tumor burden. The primary endpoint was 4-year PFS and was expected to be 70 % based on previous studies. Sixty-two patients, including 55 FL patients, were enrolled and received induction therapy with CHOP combined with rituximab (R-CHOP). Fifty-eight patients responding to R-CHOP induction received rituximab at 375 mg/m2 every 8 weeks for 2 years as for the rituximab maintenance arm in the PRIMA study. A 4-year PFS of 69.8 % was obtained (95 % confidence interval 55.9–80.0 %). Rituximab maintenance was well tolerated and common adverse events were infections, neutropenia, and/or leukopenia that were manageable with conventional supportive care. No patients died. These data were compatible with the PRIMA data. R-CHOP induction followed by rituximab is useful in Japanese patients with untreated indolent B-NHL having high tumor burden.
Clinical trial number UMIN000001191


Similar content being viewed by others

References
Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45.
van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–301.
van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28:2853–8.
Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 study. J Clin Oncol. 2009;27:1607–14.
Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA. Maximizing therapeutic benefit of rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin’s lymphoma—a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23:1088–95.
Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly × 4 schedule. Blood. 2004;103:4416–23.
Vidal L, Gafter-Gvili A, Salles G, Dreyling MH, Ghielmini M, Hsu Schmitz SF, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: an updated systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2011;103:1799–806.
Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51.
Seymour JF, Feugier P, Offner F, Lopez-Guillermo A, Belada D, Xerri L, et al. Updated 6 year follow-up of the PRIMA Study confirms the benefit of 2-year rituximab maintenance in follicular lymphoma patients responding to frontline immunochemotherapy. Blood. 2013;122:509 (abstract).
Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001.
Decaudin D, Lepage E, Brousse N, Brice P, Harousseau JL, Belhadj K, et al. Low-grade stage III–IV follicular lymphoma: multivariate analysis of prognostic factors in 484 patients—a study of the Groupe d’ Etude des Lymphomes de l’Adulte. J Clin Oncol. 1999;17:2499–505.
Solal-Céligny P, Lepage E, Brousse N, Tendler CL, Brice P, Haïoun C, et al. Doxorubicin-containing regimen with or without interferon alfa-2b for advanced follicular lymphomas: final analysis of survival and toxicity in the Groupe d’Etude des Lymphomes Folliculaires 86 Trial. J Clin Oncol. 1998;16:2332–8.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244.
Ogura M, Morishima Y, Kagami Y, Watanabe T, Itoh K, Igarashi T, et al. Randomized phase II study of concurrent and sequential rituximab and CHOP chemotherapy in untreated indolent B-cell lymphoma. Cancer Sci. 2006;97:305–12.
Tobinai K, Ogura M, Itoh K, Kinoshita T, Hotta T, Watanabe T, et al. Randomized phase II study of concurrent and sequential combinations of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) chemotherapy in untreated indolent B-cell non-Hodgkin lymphoma: 7-year follow-up results. Cancer Sci. 2010;101:2579–85.
Forstpointner R, Unterhalt M, Dreyling M, Böck HP, Repp R, Wandt H, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular. Blood. 2006;108:4003–8.
Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–51.
El Kaplan, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Solal-Coligny P, Roy P, Colombat P. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65.
Behl D, Ristow K, Markovic SN, Witzig TE, Habermann TM, Colgan JP, et al. Absolute lymphocyte count predicts therapeutic efficacy of rituximab therapy in follicular lymphomas. Br J Haematol. 2007;137:409–15.
Kumagai S, Tashima M, Fujikawa J, Iwasaki M, Iwamoto Y, Sueki Y, et al. Ratio of peripheral blood absolute lymphocyte count to absolute monocyte count at diagnosis is associated with progression-free survival in follicular lymphoma. Int J Hematol. 2014;99:737–42.
Watanabe T, Tobinai K, Shibata T, Tsukasaki K, Morishima Y, Maseki N, et al. Phase II/III study of R-CHOP-21 versus R-CHOP-14 for untreated indolent B-cell non-Hodgkin’s lymphoma: JCOG 0203 trial. J Clin Oncol. 2011;29:3990–8.
Czuczman MS, Grillo-López AJ, White CA, Saleh M, Gordon L, LoBuglio AF, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–76.
Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-López AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22:4711–6.
Acknowledgments
This study was supported by Zenyaku Kogyo (Tokyo, Japan). The authors thank the patients and their families and all the investigators, including the physicians, nurses, clinical research coordinators (CRC), and laboratory technicians in the participating institutions of this multicenter trial. The authors are grateful to Drs K. Toyama (Tokyo Medical College, Tokyo, Japan), N. Horikoshi (Juntendo University School of Medicine, Tokyo, Japan), and M. Mori (Japanese Red Cross Medical Center, Tokyo, Japan) for their critical review of the clinical data as members of the Independent Data and Safety Monitoring Committee. The authors are also grateful to Drs S. Nakamura (Nagoya University Graduate School of Medicine, Nagoya, Japan), and Y. Matsuno (Hokkaido University Hospital, Sapporo, Japan) for their histopathological review as members of the Central Pathology Review Committee, and M. Matsusako (St. Luke’s International Hospital, Tokyo, Japan) for their central radiological review as members of the CT Review Committee. The authors also acknowledge K. Endo, H. Harada, T. Ito, I. Okugaito, M. Watanabe, M. Abe, T Oba, S. Kamiyama, and T. Kayo (Zenyaku Kogyo) for their help with data collection and statistical analysis.
The authors would like to thank Ms Reiko Yamaura and Dr J. Ludovic Croxford of Edanz Group Ltd. for providing English writing support, which was funded by Zenyaku Kogyo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tadahiko Igarashi and Kiyoshi Ando received research funding from Zenyaku Kogyo. Masafumi Taniwaki and Kazuhito Yamamoto received research funding from Chugai Pharmaceutical. Hirokazu Nagai received honoraria from Chugai Pharmaceutical. Yasuo Ohashi is a board member and stockholder of Statcom and received honoraria from Chugai Pharmaceutical. Kensei Tobinai received research funding from Zenyaku Kogyo and Chugai Pharmaceutical, and honoraria from Zenyaku Kogyo. The remaining authors have no conflict of interests to disclose.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s12185-016-2155-3.
About this article
Cite this article
Igarashi, T., Ogura, M., Itoh, K. et al. Japanese phase II study of rituximab maintenance for untreated indolent B-cell non-Hodgkin lymphoma with high tumor burden. Int J Hematol 104, 700–708 (2016). https://doi.org/10.1007/s12185-016-2097-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2097-9