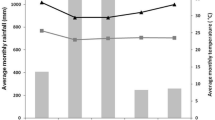
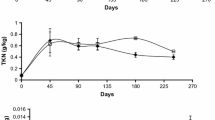
Abstract
Restoration of salt-affected soil through cultivation Chrysopogon zizanioides is a promising approach. The two way benefit of such an approach is that reclamation of salt-affected soil concomitant to improve plant growth and increased yield of essential oil produced in the plants roots. Earlier studies showed physiological changes and reduced growth of C. zizanioides under salinity. In the present study, plant growth promoting microorganisms viz. Pseudomonas monteilii, Bacillus megaterium, Azotobacter chroococcum and Rhizophagus intraradices were used as bio-inoculants for cultivation of C. zizanioides under salt-affected soil. Bio-inoculants in combination with vermicompost significantly increased the growth and productivity of C. zizanioides under salt-affected soil, and simultaneously improved soil health. When compared to control, the soil physico-chemical and biological properties of bio-inoculants treated plants was significantly improved. The reclamation of salt-affected soil was evident by the significant decrease in the level of soil pH (11.0%), electrical conductivity (23.5%), sodium adsorption ratio (15.3%), and exchangeable sodium percent (12.4%) of bio-inoculants treated plants. The improvement of soil cation exchange capacity indicated the decrease in soil salinity. Whereas increase in the microbial count (four-fold), AMF spores (447 spores), dehydrogenase (six-fold), acid (two-fold) and alkaline phosphatase (five-fold) activities in rhizosphere soil of bio-inoculant treated plants indicated the improved biological properties. A positive correlation of plant biomass production to soil organic carbon, total Kjeldahl nitrogen, available phosphorus and cation exchange capacity depicted improved nutrients content in rhizosphere soil of bio-inoculant treated plants. The findings of this study suggest that P. monteilii and R. intraradices with vermicompost can be effectively used as bio-inoculants for encouragement of phytoremediation in salt-affected soil.




Similar content being viewed by others
References
Singh K (2015) Microbial and enzyme activities of saline and sodic soils. Land Degrad De 27:706–718. https://doi.org/10.1002/ldr.2385
Singh K, Trivedi P, Singh G, Singh B, Patra DD (2016) Effect of different leaf litters on carbon, nitrogen and microbial activities of sodic soils. Land Degrad Dev 27:1215–1226. https://doi.org/10.1002/ldr.2313
Chinchmalatpure AR, Bardharr G, Nayak AK, Rao GG, Chaudhari SK, Sharma DK (2014) Effect of sodium adsorption ratio with different electrolyte concentrations on saturated hydraulic conductivity of selected salt affected soils of Gujarat. J Indian Soc Soil Sci 62:1–8
Berruti A, Lumini E, Balestrini R, Bianciotto V (2016) Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front Microbiol 6:1559. https://doi.org/10.3389/fmicb.2015.01559
Belligno A, Russo M, Sardo V, Wu JY (2008) Salinity influence on soil microbial population metabolism and enzymatic activities in lysimeter-grown Olea europaea and Nicotiana glauca. In: Biosaline agriculture and high salinity tolerance. Springer, pp 131–139. https://doi.org/10.1007/978-3-7643-8554-5_13
Ezeaku PI, Ene J, Shehu JA (2015) Application of different reclamation methods on salt affected soils for crop production. Am J Exp Agric 9:1–11. https://doi.org/10.9734/AJEA/2015/17187
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26:115–124. https://doi.org/10.1016/j.copbio.2013.12.004
Bimpong IK, Manneh B, Sock M, Diaw F, Amoah NKA, Ismail AM, Gregorio G, Singh RK, Wopereis M (2016) Improving salt tolerance of lowland rice cultivar ‘Rassi’ through marker-aided backcross breeding in West Africa. Plant Sci 242:288–299. https://doi.org/10.1016/j.plantsci.2015.09.020
Rojas-Tapias D, Moreno-Galván A, Pardo-Díaz S, Obando M, Rivera D, Bonilla R (2012) Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl Soil Ecol 61:264–272. https://doi.org/10.1016/j.apsoil.2012.01.006
Leifheit EF, Verbruggen E, Rillig MC (2015) Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biol Biochem 81:323–328. https://doi.org/10.1016/j.soilbio.2014.12.003
Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann A (2015) Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol 205:1385–1388. https://doi.org/10.1111/nph.13045
Singh K, Pandey VC, Singh RP (2013) Cynodon dactylon: an efficient perennial grass to revegetate sodic lands. Ecol Eng 54:32–38. https://doi.org/10.1016/j.ecoleng.2013.01.007
Maffei ME (2010) Sites of synthesis, biochemistry and functional role of plant volatiles. S Afr J Bot 76:612–631. https://doi.org/10.1016/j.sajb.2010.03.003
Champagnat P, Figueredo G, Chalchat JC (2006) A study on the composition of commercial Vetiveria zizanioides oils from different geographical origins. J Essent Oil Res 18:416–422. https://doi.org/10.1080/10412905.2006.9699129
Liu WG, Liu JX, Yao ML, Ma QF (2016) Salt tolerance of a wild ecotype of vetiver grass (Vetiveria zizanioides L.) in southern China. Bot Stud 57:27. https://doi.org/10.1186/s40529-016-0142-x
Cuong DC, Minh VV, Truong P (2015) Effects of sea water salinity on the growth of vetiver grass (Chrysopogon zizanioides L.). In: 6th international conference on vetiver (ICV6) Da Nang, pp 1–10
Zhou Q, Yu BJ (2009) Accumulation of inorganic and organic osmolytes and their role in osmotic adjustment in NaCl-stressed vetiver grass seedlings. Russ J Plant Physiol 56:678–685. https://doi.org/10.1134/S1021443709050148
Gupta PK (2007) Methods in environmental analysis: water, soil and air, 2nd edn. AgroBios, Jodhpur
Singh R, Divya S, Awasthi A, Kalra A (2012) Technology for efficient and successful delivery of vermicompost colonized bioinoculants in Pogostemon cablin (patchouli) Benth. World J Microbiol Biotechnol 28:323–333. https://doi.org/10.1007/s11274-011-0823-2
Singh R, Singh R, Soni SK, Singh SP, Chauhan UK, Kalra A (2013) Vermicompost from biodegraded distillation waste improves soil properties and essential oil yield of Pogostemon cablin (patchouli) Benth. Appl Soil Ecol 70:48–56. https://doi.org/10.1016/j.apsoil.2013.04.007
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis: chemical and microbiological properties. Agronomy, monogroaph, vol 9.2. ASA, SSSA, Madison. https://doi.org/10.2134/agronmonogr9.2.frontmatter
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161. https://doi.org/10.1016/S0007-1536(70)80110-3
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular infection in roots. New Phytol 84:489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining organic carbon in soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–263
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. United States Department of Agriculture, Washington
Hanway JJ, Heidel H (1952) Soil analyses methods as used in Iowa state college soil testing laboratory. Iowa Agric 57:1–31
Daniels GW, Skipper HD (1982) Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck NC (ed) Methods and principles of mycorrhizal research. American Phytopathology Society, St. Pau, pp 29–35
Tabatabai MA (1982) Methods of soil analysis. In: Page AL, Frene JR, Miller RH (eds) Chemical and microbiological properties (Part II). ASA-CSSA-SSSA, Madison, pp 501–538
Gopinathan R, Prakash M (2014) Effect of vermicompost enriched with bio-fertilizers on the productivity of tomato (Lycopersicum esculentum mill.). Int J Curr Microbiol Appl Sci 3:1238–1245
Pathma J, Sakthivel N (2012) Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus 1:26. https://doi.org/10.1186/2193-1801-1-26
Krishnamoorthy R, Kim K, Subramanian P, Senthilkumar M, Anandham R, Sa T (2016) Arbuscular mycorrhizal fungi and associated bacteria isolated from salt-affected soil enhances the tolerance of maize to salinity in coastal reclamation soil. Agric Ecosyst Environ 231:233–239. https://doi.org/10.1016/j.agee.2016.05.037
McNear DH Jr (2013) The rhizosphere-roots, soil and everything in between. Nat Educ Knowl 4:1
Babu G, Reddy MS (2011) Influence of arbuscular mycorrhizal fungi on the growth and nutrient status of bermudagrass grown in alkaline bauxite processing residue. Environ Pollut 159:25–29. https://doi.org/10.1016/j.envpol.2010.09.032
Koranda M, Schnecker J, Kaiser C, Fuchslueger L et al (2011) Microbial processes and community composition in the rhizosphere of European beech: the influence of plant C exudates. Soil Biol Biochem 43:551–558. https://doi.org/10.1016/j.soilbio.2010.11.022
Kumar N, Khader JBMA, Rangaswami P, Irulappan I (2010) Introduction to spices, plantation crops, medicinal and aromatic plants. Oxford and IBH Publishing, New Delhi, pp 22.10–22.16
Tripathi AK, Nagarajan T, Verma SC, Rudulier DL (2002) Inhibition of biosynthesis and activity of nitrogenase in Azospirillum brasilense Sp7 under salinity stress. Curr Microbiol 44:363–367. https://doi.org/10.1007/s00284-001-0022-8
Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ, Lee KE, Kim JH (2014) Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol 54:427–433. https://doi.org/10.1007/s12088-014-0476-6
Battini F, Grønlund M, Agnolucci M, Giovannetti M, Jakobsen I (2017) Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci Rep 7:4686. https://doi.org/10.1038/s41598-017-04959-0
Wang Y, Wang M, Li Y, Wu A, Huang J (2018) Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 13:e0196408. https://doi.org/10.1371/journal.pone.0196408
Balliu A, Sallaku G, Rewald B (2015) AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7:15967–15981
Evelin H, Giri B, Kapoor R (2012) Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22:203–217. https://doi.org/10.1007/s00572-011-0392-0
Al-Karaki GN (2017) Effects of mycorrhizal fungi inoculation on green pepper yield and mineral uptake under irrigation with saline water. Adv Plants Agric Res 6:00231. https://doi.org/10.15406/apar.2017.06.00231
Utobo EB, Tewari L (2015) Soil enzymes as bioindicators of soil ecosystem status. Appl Ecol Environ Res 13:147–169. https://doi.org/10.15666/aeer/1301_147169
Das SK, Varma A (2011) Role of enzymes in maintaining soil health. In: Shukla G, Varma A (eds) Soil enzymology. Soil biology, vol 22. Springer, Berlin
Badda N, Yadav K, Kadian N, Aggarwal A (2013) Impact of arbuscular mycorrhizal fungi with Trichoderma viride and Pseudomonas fluorescens on growth enhancement of genetically modified Bt Cotton (Bacillus thuringiensis). J Nat Fibers 10:309–325. https://doi.org/10.1080/15440478.2013.791913
Deveau A, Labbé J (2016) Mycorrhiza helper bacteria. In: Martin F (ed) Molecular mycorrhizal symbiosis. Wiley, Hoboken, pp 437–450. https://doi.org/10.1002/9781118951446.ch24
Daynes CN, Field DJ, Saleeba JA, Cole MA, McGee PA (2013) Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biol Biochem 57:683–694. https://doi.org/10.1016/j.soilbio.2012.09.020
Acknowledgements
The authors are thankful to director CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow (India) for providing all the necessary facilities under institutional network Project-BSC0110.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Geetu Singh: AcSIR-CIMAP PhD student
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pankaj, U., Singh, D.N., Singh, G. et al. Microbial Inoculants Assisted Growth of Chrysopogon zizanioides Promotes Phytoremediation of Salt Affected Soil. Indian J Microbiol 59, 137–146 (2019). https://doi.org/10.1007/s12088-018-00776-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-018-00776-9