Abstract
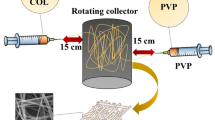
Recently electrospun scaffolds show excellent response in cell adhesion, growth, and tissue healing in comparison with other techniques. So in this study, PCL and PCL/DCPD scaffolds were designed and prepared with electrospinning. The electrospun scaffolds were characterized by scanning electron microscope with X-ray elemental analysis, atomic force microcopy, differential scanning calorimetry, and contact angle analysis for optimizing the effective parameters. Fiber formation with uniform diameter and bead-free structure was obtained. Scaffold surface roughness increased from 100 nm for PCL to 440 nm for PCL/DCPD. DSC analysis showed the effects of DCPD on thermal stability of composite scaffold and the results of contact angle evaluation indicate improved hydrophilicity and ability of water absorption of PCL/DCPD composite fibers as compared to PCL fibers. MTT assay indicated lack of toxicity for human gingival fibroblast (HGF) cells after cell seeding on scaffold. Also, the composite scaffold can improve cell viability by helping their growth on its surface. So it can be concluded that by engineering the electrospinning parameters we can fabricate a PCL/DCPD composite scaffold for tissue engineering applications.







Similar content being viewed by others
References
Ghiasi, M. S., Chen, J., Vaziri, A., Rodriguez, E. K., & Nazarian, A. (2017). Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Reports, 6, 87–100. https://doi.org/10.1016/j.bonr.2017.03.002.
Carpinteri, A., Berto, F., Fortese, G., Ronchei, C., Scorza, D., & Vantadori, S. (2017). Modified two-parameter fracture model for bone. Engineering Fracture Mechanics, 174, 44–53. https://doi.org/10.1016/j.engfracmech.2016.11.002.
Wang, W., & Yeung, K. (2017). Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioactive Materials, 2(4), 224–247. https://doi.org/10.1016/j.bioactmat.2017.05.007.
Nasiri, F., Ajeli, S., Semnani, D., Jahanshahi, M., & Emadi, R. (2018). Design, fabrication and structural optimization of tubular Carbon/Kevlar®/PMMA/Graphene nanoplates composite for bone fixation prosthesis. Biomedical Materials. https://doi.org/10.1088/1748-605X/aab8d6.
Tavakol, S., Nikpour, M. R., Amani, A., Soltani, M., Rabiee, S. M., Rezayat, S. M., & Chen, P. (2013). Bone regeneration based on nano-hydroxyapatite and hydroxyapatite/chitosan nanocomposites: An in vitro and in vivo comparative study. Journal of Nanoparticle Research, 15(1), 1373–1378. https://doi.org/10.1007/s11051-012-1373-8.
Hollister, S. J. (2005). Porous scaffold design for tissue engineering. Nature Materials, 4, 518–524. https://doi.org/10.1038/nmat1421.
Leung, V., & Ko, F. (2011). Biomedical applications of nanofibers. Polymers for Advanced Technologies, 22, 350–365. https://doi.org/10.1002/pat.1813.
Beachley, V., & Wen, X. (2010). Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Progress in Polymer Science, 35, 868–892. https://doi.org/10.1016/j.progpolymsci.2010.03.003.
Huang, Z.M., Zhang, Y.Z., Kotaksi, M., & Ramakrishna, S. (2003). A review on polymer nanofibers by electrospinning and their application in nanocomposites. Composites Science and Technology, 63, 2223–2253. https://doi.org/10.1016/S0266-3538(03)00178-7.
Teo, W. E., & Ramakrishna, S. (2006). A review on electrospinning design and nanofiber assemblies. Nanotechnology, 17, 89–106. https://doi.org/10.1088/0957-4484/17/14/R01.
Villarreal-Gómez, L. J., Cornejo-Bravo, J. M., Graziano, R. V., & Grande, D. (2016). Electrospinning as a powerful technique for biomedical applications: A critically selected survey. Journal of Biomaterials Science, Polymer Edition, 27, 157–176. https://doi.org/10.1080/09205063.2015.1116885.
Liu, J., & Wang, P. (2017). Electrospinning based functional nanofibers for biomedical applications. Journal of Biomaterials and Tissue Engineering, 7(7), 511–518. https://doi.org/10.1166/jbt.2017.1605.
Gunatillake, P. A., Mayadunne, R., & Adhikari, R. (2006). Recent developments in biodegradable synthetic polymers. Biotechnology Annual Review., 12, 301–347. https://doi.org/10.1016/S1387-2656(06)12009-8.
Rahmani-Monfard, K., Fathi, A., & Rabiee, S. M. (2016). Three-dimensional laser drilling of polymethyl methacrylate (PMMA) scaffold used for bone regeneration. The International Journal of Advanced Manufacturing Technology, 84, 2649–2657. https://doi.org/10.1007/s00170-015-7917-1.
Siddiqui, N., Asawa, S., Birru, B., Baadhe, R., & Rao, S. (2018). PCL–based composite scaffold matrices for tissue engineering. Molecular Biotechnology, 60, 506–532. https://doi.org/10.1007/s12033-018-0084-5.
Cipitria, A., Skelton, A., Dargaville, T. R., Dalton, P. D., & Hutmacher, D. W. (2011). Design, fabrication and characterization of PCL electrospun scaffolds—a review. Journal Materials Chemistry, 21, 9419–9453. https://doi.org/10.1039/C0JM04502K.
Rabiee, S. M. (2011). Bioactive ceramics as bone morphogenetic proteins carriers. In R. Pignatello (Ed.), Biomaterials applications for nanomedicine (pp. 1–15), London: InTech.
Rabiee, S. M., Moztarzadeh, F., Salimi-Kenari, H., Solati-Hashjin, M., & Mortazavi, S. M. J. (2008). Study of biodegradable ceramic bone graft substitute. Advances in Applied Ceramics, 107, 199–202. https://doi.org/10.1179/174367607X227972.
Zhao, X., Lui, Y. S., Choo, C. K., Sow, W. T., Huang, C. L., Woei, N. K., Tan, L. P., & Loo, J. S. C. (2015). Calcium phosphate coated Keratin–PCL scaffolds for potential bone tissue regeneration. Materials Science and Engineering: C, 49, 746–753. https://doi.org/10.1016/j.msec.2015.01.084.
Chuanglong, H., Peter, Xb,J. X.,. M. (2014). Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation. Acta Biomaterialia. https://doi.org/10.1016/j.actbio.2013.08.041.
Yang, F., Wolke, J. G. C., & Jansen, J. A. (2008). Biomimetic calcium phosphate coating on electrospun poly(ɛ-caprolactone) scaffolds for bone tissue engineering. Chemical Engineering Journal, 137, 154–161. https://doi.org/10.1016/j.cej.2007.07.076.
Kouhi, M., Morshed, M., Varshosaz, J., & Fathi, M. H. (2013). Poly(ε-caprolactone) incorporated bioactive glass nanoparticles and simvastatin nanocomposite nanofibers: Preparation, characterization and in vitro drug release for bone regeneration applications. Chemical Engineering Journal, 228, 1057–1065. https://doi.org/10.1016/j.cej.2013.05.091.
Zong, H. X., Xia, X., Liang, Y. R., Dai, S. Y., Alsaedi, A., Hayat, T., Kong, F. T., & Pan, J. H. (2018). Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Materials Science and Engineering: C, 92, 1075–1091. https://doi.org/10.1016/j.msec.2017.11.007.
Wang, X. F., Ding, B., & Li, B., Y (2013). Biomimetic electrospun nanofibrous structures for tissue engineering. Materials Today, 16(6), 229–241. https://doi.org/10.1016/j.mattod.2013.06.005.
Wan, C., & Chen, B. (2011). Poly(ε-caprolactone)/graphene oxide biocomposites: Mechanical properties and bioactivity. Biomedical Materials, 6, 1–8. https://doi.org/10.1088/1748-6041/6/5/055010.
Islam,M. D. S., &Karima,M. R.(2010).Fabrication and characterization of poly(vinyl alcohol)/alginate blend nanofibers by electrospinning method. Colloids and Surfaces A: Physicochem, Engineering Aspects,366,135–140.https://doi.org/10.1016/j.colsurfa.2010.05.038.
Guoyou, H., Fei, L., Xin, Z., Yufei, M., Yuhui, L., Min, L., Guorui, J., Tian, J. L., Guy, M., Genin, & Feng, X. (2017). Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chemical Reviews, 117, 12764–12850. https://doi.org/10.1021/acs.chemrev.7b00094.
Das, R. K., Gocheva, V., Hammink, R., Zouani, O. F., & Rowan, A. E. (2016). Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nature Materials, 15, 318–325.
Wang, H., Abhilash, A. S., Christopher, C., Wells, S., R. G., & Shenoy, V. B. (2014). Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophysical Journal, 107, 2592–2603. https://doi.org/10.1016/j.bpj.2014.09.044.
Babaei, B., Davarian, A., Lee, S. L., Pryse, K. M., McConnaughey, W.B., Elson, E. L., & Genin, G. M. (2016). Remodeling by fibroblasts. Acta Biomaterialia, 37, 28–37. https://doi.org/10.1016/j.actbio.2016.03.034.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taghavi, M.A., Rabiee, S.M., Jahanshahi, M. et al. Electrospun Poly-ε-Caprolactone (PCL)/Dicalcium Phosphate Dihydrate (DCPD) Composite Scaffold for Tissue Engineering Application. Mol Biotechnol 61, 345–354 (2019). https://doi.org/10.1007/s12033-019-00168-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00168-4