Abstract
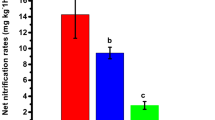
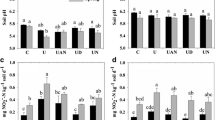
An incubation experiment was conducted to investigate the response of ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA), and the nitrification rate to the contamination of Cu, Zn, and Cd in two New Zealand grassland soils. The soils spiked with different concentrations of Cu (20 and 50 mg kg−1), Zn (20 and 50 mg kg−1), and Cd (2 and 10 mg kg−1) were incubated for 14 days and then treated with 500 mg kg−1 urine-N before continuing incubation for a total of 115 days. Soils were sampled at intervals throughout the incubation. The nitrification rate in soils at each sampling period was determined, and the abundance of AOB and AOA was measured by real-time quantification polymerase chain reaction (qPCR) assay of the amoA gene copy numbers. The results revealed that moderate trace metal stress did not significantly affect the abundance of AOB and AOA in the two soils, probably due to the high organic matter content of the soils which would have reduced the toxic effect of the metals. Nitrification rates were much greater and the observable nitrification period was much shorter in the dairy farm (DF) soil, in which the AOB and AOA abundances were greater than those of the mixed cropping farm (MF) soil. AOB were shown to grow under high nitrogen conditions, whereas AOA were shown to grow under low N environments, with different metal concentrations. Therefore, nitrogen status rather than metal applications was the main determining factor for AOB and AOA growth in the two soils studied.




Similar content being viewed by others
References
Bermudez GMA, Moreno M, Invernizzi R, Plá R, Pignata ML (2009) Heavy metal pollution in topsoils near a cement plant: the role of organic matter and distance to the source to predict total and HCl-extracted heavy metal concentrations. Chemosphere 78(4):375–381
Cao H, Li M, Hong Y, Gu J (2011) Diversity and abundance of ammonia-oxidizing archaea and bacteria in polluted mangrove sediment. Syst Appl Microbial 34(7):513–523
Dancer WS, Peterson LA, Chesters G (1973) Ammonification and nitrification of N as influenced by soil pH and previous N treatments. Soil Sci Soc Am J 37(1):67–69
Di HJ, Cameron KC (2016) Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: a review. J Soils Sediments 16:1401–1420. doi:10.1007/s11368-016-1403-8
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callagan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geosci 2(2):621–624
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callagan M, Bowatte S, He JZ (2010) Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72(3):386–394
Di HJ, Cameron KC, Podolyan A, Robinson A (2014) Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol Biochem 73:59–68
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. P Natl Acad Sci 102(41):14683–14688
Frey B, Pesaro M, Rüdt A, Widmer F (2008) Resilience of the rhizosphere Pseudomonas, and ammonia-oxidizing bacterial populations during phytoextraction of heavy metal polluted soil with poplar. Environ Microb 10(6):1433–1449
Gao J, Fan X, Wu G, Li T, Pan K (2015) Changes of abundance and diversity of ammonia-oxidizing archaea (AOA) and bacteria (AOB) in three nitrifying bioreactors with different ammonia concentrations. Desalin Water Treat:1–13
Haferburg G, Kothe E (2007) Microbes and metals: interactions in the environment. J Basic Microb 47(6):453–467
Harris KR, Cheng TC (2004) Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Appl Environ Microb 70(2):1008–1016
He J, Shen J, Zhang L, Zhu Y, Zheng Y, Xu M, Di H (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 09:2364
Khan S, Hesham EL, Qiao M, Rehman S, He JZ (2010) Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut R 17(2):288–296
Klotz MG, Alzerreca J, Norton JM (1997) A gene encoding a membrane protein exists upstream of the amoA/amoB, genes in ammonia oxidizing bacteria: a third member of the amo, operon? FEMS Microbiol Lett 150(1):65–73
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55(1):485–529
Kris B, Jelle M, Erik S (2005) Toxicity of heavy metals in soil assessed with various soil microbial and plant growth assays: a comparative study. Environ Toxicol Chem 24(3):634–640
Li X, Zhu YG, Cavagnaro TR, Chen M, Sun J, Chen X, Qiao M (2009a) Do ammonia-oxidizing archaea respond to soil cu contamination similarly as ammonia-oxidizing bacteria? Plant Soil 324(1–2):209–217
Li F, Zheng YM, He JZ (2009b) Microbes influence the fractionation of arsenic in paddy soils with different fertilization regimes. Sci Total Environ 407(8):2631–2640
Liu YR, Zheng YM, Shen JP, Zhang LM, He JZ (2010) Effects of mercury on the activity and community composition of soil ammonia oxidizers. Environ Sci Pollut R 17(6):1237–1244
Mertens J, Springael D, Troyer ID, Cheyns K, Wattiau P, Smollders E (2006) Long-term exposure to elevated zinc concentrations induced structural changes and zinc tolerance of the nitrifying community in soil. Environ Microbiol 8(12):2170–2180
Nishio T, Fujimoto T (1990) Kinetics of nitrification of various amounts of ammonium added to soils. Soil Biol Bioch 22(1):51–55
Ollivier J, Wanat N, Austruy A, Hitmi A, Joussein E, Welzl G, Munch JC, Schloter M (2012) Abundance and diversity of ammonia-oxidizing prokaryotes in the root–rhizosphere complex of Miscanthus × giganteus grown in heavy metal-contaminated soils. Microb Ecol 64(4):1038–1046
Oved T, Shaviv A, Goldrath T, Mandelbaum RT, Minz D (2001) Influence of effluent irrigation on community composition and function of ammonia-oxidizing bacteria in soil. Appl Environ Microb 67(8):3426–3433
Philippot L, Hallin S (2005) Finding the missing link between diversity and activity using denitrifying bacteria as a model functional community. Curr Opin Microbiol 8(3):234–239
Prosser JI, Embley TM (2002) Cultivation-based and molecular approaches to characterisation of terrestrial and aquatic nitrifiers. Anton Leeuw 81(81):165–179
Qu J, Ren G, Chen B, Fan J, Yong E (2011) Effects of lead and zinc mining contamination on bacterial community diversity and enzyme activities of vicinal cropland. Environ Monit Asses 182(1–4):597–606
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microb 63(12):E61–E62
Schleper C, Jurgens G, Jonuscheit M (2005) Genomic studies of uncultivated archaea. Nat Rev Microbiol 3(6):479–488
Stefanowicz AM, Niklińska M, Laskowski R (2008) Metals affect soil bacterial and fungal functional diversity differently. Environ Toxicol Chem 27(3):591–598
Stephen JR, Chang YJ, Macnaughton SJ, Kowalchuk GA, Leung KT, Flemming CA, White DC (1999) Effect of toxic metals on indigenous soil beta-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl Environ Microb 65(1):95–101
Subrahmanyam G, Shen JP, Liu YR, Archana G, He JZ (2014) Response of ammonia-oxidizing archaea and bacteria to long-term industrial effluent-polluted soils, Gujarat, Western India. Environ Monit Asses 186(7):4037–4050
Tian M, Yang Y, Huang Q, Zhang Z, He J, Yu H (2014) Effects of pH on release of heavy metal in sintered brick. Chinese J of Environ Eng 8(5):2057–2062
Valentine DL (2007) Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat Rev Microbiol 5(4):316–323
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, De Vos P, Verstraete W, Boon N (2009) Initial community evenness favours functionality under selective stress. Nature 458(7238):623–626
Wu J, Zhang H, He PJ, Shao LM (2011) Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis. Water Res 45(4):1711–1719
Acknowledgments
This study was supported by the Natural Science Foundation of China (No. 41271275) and the Introduction of International Advanced Agricultural Science and Technology Plan of Ministry of Agriculture “948 Program” (No. 2014-Z22). We would like to thank Jie Lei of Lincoln University for the technical support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Robert Duran
Rights and permissions
About this article
Cite this article
Wang, P., Di, H.J., Cameron, K.C. et al. The response of ammonia-oxidizing microorganisms to trace metals and urine in two grassland soils in New Zealand. Environ Sci Pollut Res 24, 2476–2483 (2017). https://doi.org/10.1007/s11356-016-8030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8030-1