Abstract
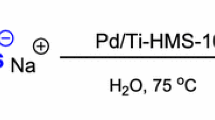
For the first time it was established that the catalytic hydrogenation of 2,4,6-trinitrobenzoic acid to 1,3,5-triaminobenzene can proceed via the formation of aromatic hydroxyamines and cyclohexane-1,3,5-trione trioxime. As a result of aqueous-phase hydrogenation of sodium salt of 2,4,6-trinitrobenzoic acid in the presence of 5%Pd/Sibunit catalyst at a temperature of 323 K and pressure of 0.5 MPa, a trioxime in high yield (about 70 %) was obtained. Due to high selectivity to cyclohexane-1,3,5-trione trioxime the catalytic hydrogenation of sodium salt of 2,4,6-trinitrobenzoic acid can be considered as a new method for its synthesis.
Similar content being viewed by others
References
M. Saytzeff, J. Prakt. Chem., 1872, 6, 128.
H. Kolbe, J. Prakt. Chem., 1871, 4, 418.
S. Nishimura, Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, John Wiley and Sons, New York, 2001, p. 315.
A. V. Lefedova, A. V. Ulitin, A. V. Barbov, Ros. Khim. Zh., 2006, 50, 123 [Mendeleev Chem. J., 2006, 50].
A. I. Kozlov, V. L. Zbarsky, Ros. Khim. Zh., 2006, 50, 131 [Mendeleev Chem. J., 2006, 50].
H.-U. Blaser, H. Steiner, M. Studer, Chem CatChem, 2009, 1, 210.
P. Lara, K. Philippot, Catal. Sci. Technol., 2014, 4, 2445.
A. S. Travis, in The Chemistry of Anilines, Part 1, Ed. Z. Rappoport, John Wiley and Sons, Chichester, 2007, p. 715.
P. Wanninger, Chem. Unserer Zeit, 1995, 29, 135.
A. F. Litvin, V. Z. Sharf, Ros. Khim. Zh., 2000, 44, 90 [Men deleev Chem. J., 2000, 44].
V. L. Zbarsky, V. F. Zhilin, Toluol i ego nitroproizvodnye [Toluene and its Nitroderivatives], Editorial URSS, Moscow, 2000, p. 272 (in Russian).
A. L. Rusanov, L. G. Komarova, D. Yu. Likhachev, S. A. Shevelev, V. A. Tartakovsky, Russ. Chem. Rev., 2003, 72, 899.aaa
M. L. Kastens, J. F. Kaplan, Ind. Eng. Chem., 1950, 42, 402.aaa
GB Pat. 1106088; Chem. Abstr., 1968, 69, 35731.
US Pat. 3678114; Chem. Abstr., 1972, 77, 61517.
Y. Chen, D. Zhao, Ranliao Gongye, 2001, 38, 24.
Q.-H. Yang, Y.-S. Feng, L. Chen, Zhongguo Yiyao Gongye Zazhi, 2005, 36, 735.
Z.-L. Yao, R.-B. Wang, H.-L. Liu, Yingyong Huagong, 2009, 38, 1153.
V. A. Kuz’mina, Yu. A. Lapina, S. V. Sysolyatin, Polzunovskiy vestnik [Polzunovsky Vestnik], 2008, No. 3, 129 (in Russian).
G. S. Samuelsen, V. L. Garik, G. B. L. Smith, J. Am. Chem. Soc., 1950, 72, 3872.
DE Pat. 102358; Chem. Zentralbl., 1899, 1, 1263.
J. Phillips, A. Lowy, Trans. Electrochem. Soc., 1937, 71, 493.
R. W. Lewis, O. W. Brown, Trans. Electrochem. Soc., 1943, 84, 135.
K. E. Malterud, J. Prakt. Chem., 1979, 321, 175.
Pat. RF No. 2337092; Bull. Izobr. [Bulletin of Inventions], 2008, A 30 (in Russian).
Pat. RF No. 2396245; Bull. Izobr. [Bulletin of Inventions], 2010, A 22 (in Russian).
V. P. Ananikov, L. L. Khemchyan, Yu. V. Ivanova, V. I. Bukhtiyarov, A. M. Sorokin, I. P. Prosvirin, S. Z. Vatsadze, A. V. Medved’ko, V. N. Nuriev, A. D. Dilman, V. V. Levin, I. V. Koptyug, K. V. Kovtunov, V. V. Zhivonitko, V. A. Likholobov, A. V. Romanenko, P. A. Simonov, V. G. Nenajdenko, O. I. Shmatova, V. M. Muzalevskiy, M. S. Nechaev, A. F. Asachenko, O. S. Morozov, P. B. Dzhevakov, S. N. Osipov, D. V. Vorobyeva, M. A. Topchiy, M. A. Zotova, S. A. Ponomarenko, O. V. Borshchev, Yu. N. Luponosov, A. A. Rempel, A. A. Valeeva, A. Yu. Stakheev, O. V. Turova, I. S. Mashkovsky, S. V. Sysolyatin, V. V. Malykhin, G. A. Bukhtiyarova, A. O. Terent’ev, I. B. Krylov, Russ. Chem. Rev., 2014, 83, 885.
O. B. Belskaya, R. M. Mironenko, V. A. Rodionov, V. P. Talsi, S. V. Sysolyatin, V. A. Likholobov, Proc. Int. Conf. «12th Eur. Congress on Catalysis–EuropaCat-XII» (Kazan, August 30–September 4, 2015), Boreskov Institute of Catalysis, Novosibirsk, 2015, p. 2027.
Yu. I. Yermakov, V. F. Surovikin, G. V. Plaksin, V. A. Semikolenov, V. A. Likholobov, L. V. Chuvilin, S. V. Bogdanov, React. Kinet. Catal. Lett., 1987, 33, 435.
US Pat. 4978649; Chem. Abstr., 1990, 112, 80449.
G. V. Plaksin, Khimiya Ustoich. Razvitiya [Chemistry for Sustainable Development], 2001, 9, 609 (in Russian).
P. A. Simonov, S. Yu. Troitskii, V. A. Likholobov, Kinet. Catal., 2000, 41, 255 [Ainetika i kataliz, 2000, 41, 281].
R. A. Mironenko, A. B. Bel’skaya, A. V. Lavrenov, V. A. Likholobov, Khimiya Ustoich. Razvitiya [Chemistry for Sustainable Development], 2015, 23, 645 (in Russian).
H. T. Clarke, W. W. Hartman, Org. Synth., 1922, 2, 95.
C. Le Cocq, J.-Y. Lallemand, J. Chem. Soc., Chem. Commun., 1981, 150.
W. Chuntiag, V. G. Dorokhov, G. A. Boiko, B. S. Bal’zhinimaev, V. V. Barelko, Dokl. Chem. (Engl. Transl.), 2005, 402, 111 [Dokl. Akad. Nauk, 2005, 402, 503].
V. G. Dorokhov, V. I. Savchenko, Proc. of the Int. Conf. on Chemical Technology, KhA’07 (Moscow, June 17–23, 2007), LENAND, Moscow, 2007, p. 383 (in Russian).
B.-S. Liao, S.-T. Liu, J. Org. Chem., 2012, 77, 6653.
I. Arai, Y. Sei, I. Muramatsu, J. Org. Chem., 1981, 46, 4597.
J. C. Bottaro, R. Malhotra, A. Dodge, Synthesis, 2004, 499 41. Int. Pat. Appl. WO 9112231; Chem. Abstr., 1991, 115, 255830.
K. B. van Gelder, J. K. Damhof, P. J. Kroijenga, K. R. Westerterp, Chem. Eng. Sci., 1990, 45, 3159.
V. I. Savchenko, V. G. Dorokhov, Alternativnaya energetika i ekologiya [Alternative Energy and Ecology], 2012, No. 12, 77 (in Russian).
F. Lions, K. V. Martin, J. Am. Chem. Soc., 1957, 79, 1572.
O. R. Klyuchnikov, F. G. Khairutdinov, Russ. Chem. Bull. (Int. Ed.), 2004, 53, 1133 [Izv. Akad. Nauk, Ser. Khim., 2004, 1087].
Pat. RF No. 2243962; Bull. Izobr. [Bulletin of Inventions] 2005, No. 1 (in Russian).
A. Baeyer, Ber. Deutsch. Chem. Ges., 1886, 19, 159.
V. G. Dorokhov, V. I. Savchenko, Proc. of the Int. Conf. on Chemical Technology, KhT’07 (Moscow, June 17–23, 2007), LENAND, Moscow, 2007, p. 383 (in Russian).
B.-S. Liao, S.-T. Liu, J. Org. Chem., 2012, 77, 6653.
I. Arai, Y. Sei, I. Muramatsu, J. Org. Chem., 1981, 46, 4597.
J. C. Bottaro, R. Malhotra, A. Dodge, Synthesis, 2004, 499 41. Int. Pat. Appl. WO 9112231; Chem. Abstr., 1991, 115, 255830.
K. B. van Gelder, J. K. Damhof, P. J. Kroijenga, K. R. Westerterp, Chem. Eng. Sci., 1990, 45, 3159.
V. I. Savchenko, V. G. Dorokhov, Alternativnaya energetika i ekologiya [Alternative Energy and Ecology], 2012, No. 12, 77 (in Russian).
F. Lions, K. V. Martin, J. Am. Chem. Soc., 1957, 79, 1572.
O. R. Klyuchnikov, F. G. Khairutdinov, Russ. Chem. Bull. (Int. Ed.), 2004, 53, 1133 [Izv. Akad. Nauk, Ser. Khim., 2004, 1087].
Pat. RF No. 2243962; Bull. Izobr. [Bulletin of Inventions] 2005, No. 1 (in Russian).
A. Baeyer, Ber. Deutsch. Chem. Ges., 1886, 19, 159.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Professor G. V. Plaksin.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1535—1540, June, 2016.
Rights and permissions
About this article
Cite this article
Mironenko, R.M., Belskaya, O.B., Talzi, V.P. et al. An unusual reduction route of 2,4,6-trinitrobenzoic acid under conditions of aqueous-phase hydrogenation over Pd/Sibunit catalyst. Russ Chem Bull 65, 1535–1540 (2016). https://doi.org/10.1007/s11172-016-1479-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1479-8