Abstract
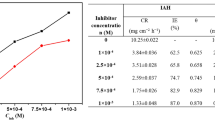
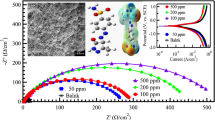
Mild steel corrosion in HCl solution is an example of corrosion in acidic mediums. The ongoing research efforts to develop novel environmentally friendly corrosion inhibitors raise questions regarding their ability to effectively protect steel from corrosion. Herein, a series of experimental studies were conducted to explain the scientific mechanism of adsorption of four hydrazone derivatives (HDZs) namely, 2-((2,3-dimethylphenyl)amino)-N′-((1E,2E)-3-phenylallylidene)benzohydrazide (HDZ1) (E)-2-((2,3-dimethylphenyl)amino)-N′-(4-hydroxybenzylidene)benzohydrazide (HDZ2) (E)-2-((2,3-dimethylphenyl)amino)-N′-(1-phenylethylidene)benzohydrazide (HDZ3) and N′-cyclohexylidene-2-((2,3-dimethylphenyl)amino)benzohydrazide (HDZ4) on mild steel (MS) in 1.0 M HCl using chemical, electrochemical and surface characterization techniques. All results show that the inhibitor molecules form a stable layer on steel surface through chemical and physical interactions. HDZs adsorption onto the steel surface was found to follow Langmuir model. Furthermore, electrochemical results demonstrated that our developed inhibitors act as mixed-type inhibitors, with HDZ1 showing the highest polarization resistance and lowest corrosion current density. X-ray diffraction and scanning electron microscope were used to study corrosion products phases and surface morphology of MS samples. Our findings provide deeper insights into understanding the interaction mechanisms of HDZs with a steel surface and can be helpful to explore novel approaches to mitigate the steel dissolution.










Similar content being viewed by others
References
A.K. Singh, E.E. Ebenso, Res. Chem. Intermed. 39, 1823 (2013)
Z. Mohammadi, M. Rahsepar, Res. Chem. Intermed. 44, 2139 (2018)
K. Adardour, R. Touir, Y. Ramli, R.A. Belakhmima, M.E. Touhami, C.K. Mubengayi, H.E. Kafsaoui, E.M. Essassi, Res. Chem. Intermed. 39, 1843 (2013)
I.B. Obot, N.O. Obi-Egbedi, E.E. Ebenso, A.S. Afolabi, E.E. Oguzie, Res. Chem. Intermed. 39, 1927 (2013)
A.K. Singh, S. Khan, A. Singh, S.M. Quraishi, M.A. Quraishi, E.E. Ebenso, Res. Chem. Intermed. 39, 1191 (2013)
B. Fan, H. Hao, B. Yang, Y. Li, Res. Chem. Intermed. 44, 5711 (2018)
S. Rollas, S.G. Küçükgüzel, Molecules 12, 1910 (2007)
G. Verma, A. Marella, M. Shaquiquzzaman, M. Akhtar, M.R. Ali, M.M. Alam, J. Pharm. Bioallied Sci. 6, 69 (2014)
M. Antonio, R.M. Maggio, J. Pharm. Biomed. Anal. 149, 603 (2018)
Y. Joo, H.-S. Kim, R.-S. Woo, C.H. Park, K.-Y. Shin, J.-P. Lee, K.-A. Chang, S. Kim, Y.-H. Suh, Mol. Pharmacol. 69, 76 (2006)
S.K. Mohamed, J.T. Mague, M. Akkurt, A.F. Mohamed, M.R. Albayati, Acta Crystallogr. Sect. E Crystallogr. Commun. 71, o957 (2015)
A. Almasirad, M. Tajik, D. Bakhtiari, A. Shafiee, M. Abdollahi, M.J. Zamani, R. Khorasani, H. Esmaily, J Pharm Pharm Sci 8, 419 (2005)
H. Nawaz, M. Khawar Rauf, M. Ebihara, A. Badshah, Acta Crystallogr. Sect. E: Struct. Rep. Online 63, o1658 (2007)
T. Aboul-Fadl, H.A. Abdel-Aziz, A. Kadi, A. Bari, P. Ahmad, T. Al-Samani, S.W. Ng, Molecules 16, 3544 (2011)
J. Scully, R. Baboian, Standard Practice for Laboratory Immersion Corrosion Testing of Metals, vol. 110 (ASTM, Philadelphia, 1995)
Z. Salarvand, M. Amirnasr, M. Talebian, K. Raeissi, S. Meghdadi, Corros. Sci. 114, 133 (2017)
ASTM Committee G-1 on Corrosion of Metals, Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens (ASTM International, West Conshohocken, 2011)
A.S. Fouda, T.F. Alsawy, E.S. Ahmed, B.S. Abou-elmagd, Res. Chem. Intermed. 39, 2641 (2013)
S. Belkaid, K. Tebbji, A. Mansri, A. Chetouani, B. Hammouti, Res. Chem. Intermed. 38, 2309 (2012)
A. Singh, J.N. Avyaya, E.E. Ebenso, M.A. Quraishi, Res. Chem. Intermed. 39, 537 (2013)
A. Mansri, B. Bouras, B. Hammouti, I. Warad, A. Chetouani, Res. Chem. Intermed. 39, 1753 (2013)
H. Lgaz, O. Benali, R. Salghi, S. Jodeh, M. Larouj, O. Hamed, M. Messali, S. Samhan, M. Zougagh, H. Oudda, Pharma Chem. 8, 172 (2016)
R. Fuchs-Godec, Colloids Surf. Physicochem. Eng. Asp. 280, 130 (2006)
A. Chetouani, B. Hammouti, T. Benhadda, M. Daoudi, Appl. Surf. Sci. 249, 375 (2005)
F. Bentiss, F. Gassama, D. Barbry, L. Gengembre, H. Vezin, M. Lagrenée, M. Traisnel, Appl. Surf. Sci. 252, 2684 (2006)
H. Lgaz, R. Salghi, M. Larouj, M. Elfaydy, S. Jodeh, Z. Rouifi, B. Lakhrissi, H. Oudda, J. Mater. Environ. Sci. 7, 4471 (2016)
M. Messali, H. Lgaz, R. Dassanayake, R. Salghi, S. Jodeh, N. Abidi, O. Hamed, J. Mol. Struct. 1145, 43 (2017)
S.K. Saha, A. Dutta, P. Ghosh, D. Sukul, P. Banerjee, Phys. Chem. Chem. Phys. 17, 5679 (2015)
H. Lgaz, R. Salghi, S. Jodeh, B. Hammouti, J. Mol. Liq. 225, 271 (2017)
A. Singh, K.R. Ansari, J. Haque, P. Dohare, H. Lgaz, R. Salghi, M.A. Quraishi, J. Taiwan Inst. Chem. Eng. 82, 233 (2018)
K. Toumiat, Y. El Aoufir, H. Lgaz, R. Salghi, S. Jodeh, M. Zougagh, H. Oudda, Res. J. Pharm. Biol. Chem. Sci. 7, 1209 (2016)
L.O. Olasunkanmi, I.B. Obot, E.E. Ebenso, RSC Adv. 6, 86782 (2016)
M. Yadav, S. Kumar, R.R. Sinha, I. Bahadur, E.E. Ebenso, J. Mol. Liq. 211, 135 (2015)
S. Tu, X. Jiang, L. Zhou, M. Duan, H. Wang, X. Jiang, Corros. Sci. 65, 13 (2012)
R. Yıldız, T. Doğan, İ. Dehri, Corros. Sci. 85, 215 (2014)
Z. Cong, W. Li, D. Hu, Z. Lan, B. Hou, Electrochemistry 83, 262 (2015)
P. Han, W. Li, H. Tian, X. Gao, R. Ding, C. Xiong, L. Song, X. Zhang, W. Wang, C. Chen, Mater. Chem. Phys. 214, 345 (2018)
R. Solmaz, Corros. Sci. 81, 75 (2014)
R. Solmaz, E. Altunbaş, G. Kardaş, Mater. Chem. Phys. 125, 796 (2011)
L. Tang, X. Li, L. Li, G. Mu, G. Liu, Mater. Chem. Phys. 97, 301 (2006)
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under Grant Number R.G.P-1.21-38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lgaz, H., Chaouiki, A., Albayati, M.R. et al. Synthesis and evaluation of some new hydrazones as corrosion inhibitors for mild steel in acidic media. Res Chem Intermed 45, 2269–2286 (2019). https://doi.org/10.1007/s11164-018-03730-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-03730-y