Abstract
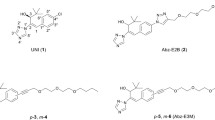
Methyl and phenyl esters of 2′,3′-PhABA and iso-2′,3′-PhABA were prepared for the biological investigation and development of practical applications. These esters exhibited excellent activity in most plant growth inhibitory assays. And, three esters were more efficient than ABA in stomatal closure. The 2′,3′-PhABA analogs and their methyl esters have good stability in hydrolysis assay, and the different lipid solubility and permeability of different esters may be one of the origins of their active selectivity for different plants and physiological processes. Furthermore, in the study of drought tolerance, all four esters had comparable activity to ABA. These results suggest that these esters were potent plant growth regulator (PGR) candidates for anti-drought. The finding that different esters have different selective bioactivity and biophysical properties indicates that these esters not only function as ABA-like PGRs but also have the possibility as potential selective pro-hormone.
Graphical abstract
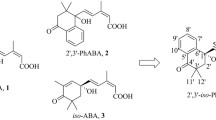
2′,3′-BenzoABA esters as PGR candidates with prolonged and selective bioactivity.








Similar content being viewed by others
References
Zeevaart JAD (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39:439–473
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42(1):55–76. https://doi.org/10.1146/annurev.pp.42.060191.000415
Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49:199–222. https://doi.org/10.1146/annurev.arplant.49.1.199
Finkelstein R (2013) Abscisic acid synthesis and response. Arabidopsis Book 11:e0166. https://doi.org/10.1199/tab.0166
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19(6):371–379. https://doi.org/10.1016/j.tplants.2014.02.001
Horton RF (1971) Stomatal opening: the role of abscisic acid. Can J Bot 49(4):583–585. https://doi.org/10.1139/b71-092
Chen THH, Gusta LV (1983) Abscisic acid-induced freezing resistance in cultured plant cells. Plant Physiol 73(1):71–75. https://doi.org/10.1104/pp.73.1.71
Zeevaart JAD, Hooykaas PJJ, Hall MA, Libbenga KR (1999) Abscisic acid metabolism and its regulation. In: Hooykaas PJJ, Hall MA, Libbenga KR (eds) Biochemistry and molecular biology of plant hormones. Elsevier Science, New York, pp 189–207. https://doi.org/10.1016/s0167-7306(08)60488-3
Chiwocha SDS, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross ARS, Kermode AR (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 35(3):405–417. https://doi.org/10.1046/j.1365-313x.2003.01800.x
Oritani T, Kiyota H (2003) Biosynthesis and metabolism of abscisic acid and related compounds. Nat Prod Rep 20(4):414–425. https://doi.org/10.1039/b109859b
Zhou R, Cutler AJ, Ambrose SJ, Galka MM, Nelson KM, Squires TM, Loewen MK, Jadhav AS, Ross AR, Taylor DC, Abrams SR (2004) A new abscisic Acid catabolic pathway. Plant Physiol 134(1):361–369. https://doi.org/10.1104/pp.103.030734
Zaharia LI, Walker-Simmon MK, Rodríguez CN, Abrams SR (2005) Chemistry of abscisic acid, abscisic acid catabolites and analogs. J Plant Growth Regul 24(4):274–284. https://doi.org/10.1007/s00344-005-0066-2
Cornforth JW, Milborrow BV, Ryback G (1965) Synthesis of (±)-Abscisin II. Nature 206:715
Cao M, Liu X, Zhang Y, Xue X, Zhou XE, Melcher K, Gao P, Wang F, Zeng L, Zhao Y, Zhao Y, Deng P, Zhong D, Zhu JK, Xu HE, Xu Y (2013) An ABA-mimicking ligand that reduces water loss and promotes drought resistance in plants. Cell Res 23(8):1043–1054. https://doi.org/10.1038/cr.2013.95
Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of Abscisic acid. Cell 126(6):1109–1120. https://doi.org/10.1016/j.cell.2006.07.034
Xu ZY, Lee KH, Dong T, Jeong JC, Jin JB, Kanno Y, Kim DH, Kim SY, Seo M, Bressan RA, Yun DJ, Hwang I (2012) A vacuolar beta-glucosidase homolog that possesses glucose-conjugated Abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 24(5):2184–2199. https://doi.org/10.1105/tpc.112.095935
Wan C, Zhang Y, Yang D, Han X, Li X, Hong L, Xiao Y, Qin Z (2015) Synthesis and biological activity of abscisic acid esters. Phytochem Lett 12:267–272. https://doi.org/10.1016/j.phytol.2015.04.015
Shingo M, Makoto S, Toru Y (1993) Abscisic acid analogue and plant growth-regulating agent. JP 05-001001. https://www19.j-platpat.inpit.go.jp/PA1/cgi-bin/PA1DETAIL?MaxCount=1000&PageCount=1000&SearchType=0&TempName=w-Lakia&MaxPage=1&DispPage=1+1000&HitCount=36&ResultId=I00419005802&CookieId=2&DetailPage=25&Language=ENG&Reserve1=DetailPaging&Reserve2=hh9II7f5d_j5dDc3cs4S&Reserve3=
Ward JL, Beale MH (1995) Caged plant hormones. Phytochemistry 38:811–816
Jones RJ, Mansfield TA (1971) Antitranspiration activity of the methyl and phenyl esters of Abscisic acid. Nature 231:331–332
Frackenpohl J, Bojack G, Baltz R, Bickers U, Busch M, Dittgen J, Franke J, Freigang J, Grill E, Gonzalez S, Helmke H, Hills MJ, Hohmann S, von Koskull-Döring P, Kleemann J, Lange G, Lehr S, Schmutzler D, Schulz A, Walther K, Willms L, Wunschel C (2018) Potent analogues of abscisic acid-identifying cyano-cyclopropyl moieties as promising replacements for the cyclohexenone headgroup. Eur J Org Chem 12:1416–1425. https://doi.org/10.1002/ejoc.201701769
Frackenpohl J, Grill E, Bojack G, Baltz R, Busch M, Dittgen J, Franke J, Freigang J, Gonzalez S, Heinemann I, Helmke H, Hills M, Hohmann S, von Koskull-Döring P, Kleemann J, Lange G, Lehr S, Müller T, Peschel E, Poree F, Schmutzler D, Schulz A, Willms L, Wunschel C (2018) Insights into the in vitro and in vivo SAR of abscisic acid-exploring unprecedented variations of the side chain via cross-coupling-mediated syntheses. Eur J Org Chem 12:1403–1415. https://doi.org/10.1002/ejoc.201701687
Balko TW, Fields SC, Webster JD (1999) Total synthesis of (±)-8′-trifluoromethyl abscisic acid. Tetrahedron Lett 40(35):6347–6351. https://doi.org/10.1016/s0040-4039(99)01206-x
Arai S, Todoroki Y, Ibaraki S, Naoe Y, Hirai N, Ohigashi H (1999) Synthesis and biological activity of 3′-chloro, -bromo, and -iodoabscisic acids, and biological activity of 3′-fluoro-8′-hydroxyabscisic acid. Phytochemistry 52(7):1185–1193. https://doi.org/10.1016/s0031-9422(99)00444-6
Ohkuma K (1965) Synthesis of some analogs of Abscisin II. Agr Biol Chem 30(5):434–437. https://doi.org/10.1080/00021369.1966.10858620
Lei B, Abrams SR, Ewan B, Gusta LV (1994) Achiral cyclohexadienone analogues of abscisic acid: synthesis and biological activity. Phytochemistry 37(2):289–296. https://doi.org/10.1016/0031-9422(94)85049-6
Todoroki Y, Nakano S, Hirai N, Mitsui T, Ohigashi H (1997) Synthesis, biological activity, and metabolism of 8′,8′,8′-trideuteroabscisic acid. Biosci Biotech Biochem 61(11):1872–1876. https://doi.org/10.1271/bbb.61.1872
Todoroki Y, Hirai N, Koshimizu K (1995) 8′,8′-Difluoro- and 8′,8′,8′-trifluoroabscisic acids as highly potent, long-lasting analogues of abscisic acid. Phytochemistry 38(3):561–568. https://doi.org/10.1016/0031-9422(94)00693-n
Todoroki Y, Hirai N, Koshimizu K (1995) Synthesis and biological activity of 1′-deoxy-1′-fluoro- and 8′-fluoroabscisic acids. Phytochemistry 40(3):633–641. https://doi.org/10.1016/0031-9422(95)00230-5
Todoroki Y, Hirai N, Koshimizu K (1994) 8′- and 9′-Methoxyabscisic acids as antimetabolic analogs of abscisic acid. Biosci Biotechnol Biochem 58(4):707–715. https://doi.org/10.1271/bbb.58.707
Rose PA, Cutler AJ, Irvine NM, Shaw AC, Squires TM, Loewen MK, Abrams SR (1997) 8′-Acetylene ABA: an irreversible inhibitor of ABA 8′-hydroxylase. Bioorg Med Chem Lett 7(19):2543–2546
Abrams SR, Rose PA, Cutler AJ, Trvine NM, Shaw AC, Squires TM, Loewen MK (1997) 8′-Methylene abscisic acid. An effective and persistent analog of Abscisic acid. Plant Physiol 114:89–97. https://doi.org/10.1104/pp.114.1.89
Nyangulu JM, Galka MM, Jadhav A, Gai Y, Graham CM, Nelson KM, Cutler AJ, Taylor DC, Banowetz GM, Abrams SR (2005) An affinity probe for isolation of Abscisic acid-binding proteins. J Am Chem Soc 127(6):1662–1664. https://doi.org/10.1021/ja0429059
Benson CL, Kepka M, Wunschel C, Rajagopalan N, Nelson KM, Christmann A, Abrams SR, Grill E, Loewen MC (2015) Abscisic acid analogs as chemical probes for dissection of abscisic acid responses in Arabidopsis thaliana. Phytochemistry 113:96–107. https://doi.org/10.1016/j.phytochem.2014.03.017
Takeuchi J, Ohnishi T, Okamoto M, Todoroki Y (2015) Conformationally restricted 3′-modified ABA analogs for controlling ABA receptors. Org Biomol Chem 13(14):4278–4288. https://doi.org/10.1039/c4ob02662d
Rajagopalan N, Nelson KM, Douglas AF, Jheengut V, Alarcon IQ, McKenna SA, Surpin M, Loewen MC, Abrams SR (2016) Abscisic acid analogues that act as universal or selective antagonists of phytohormone receptors. Biochemistry 55(36):5155–5164. https://doi.org/10.1021/acs.biochem.6b00605
Han X, Wan C, Li X, Li H, Yang D, Du S, Xiao Y, Qin Z (2015) Synthesis and bioactivity of 2′,3′-benzoabscisic acid analogs. Bioorg Med Chem Lett 25(11):2438–2441. https://doi.org/10.1016/j.bmcl.2015.03.071
Wan C, Wang M, Yang D, Han X, Che C, Ding S, Xiao Y, Qin Z (2017) Synthesis and biological activity of 2′,3′-iso-aryl-abscisic acid analogs. Molecules 22(12):2229. https://doi.org/10.3390/molecules22122229
Neises B, Steglich W (1978) Simple method for the esterification of carboxylic acids. Angew Chem Int Ed Engl 17(7):522–524. https://doi.org/10.1002/anie.197805221
Han X, Jiang L, Che C, Wan C, Lu H, Xiao Y, Xu Y, Chen Z, Qin Z (2017) Design and functional characterization of a novel abscisic acid analog. Sci Rep 7:43863. https://doi.org/10.1038/srep43863
Han X, Zhou Z, Wan C, Xiao Y, Qin Z (2013) Co(acac)2-catalyzed allylic and benzylic oxidation by tert-butyl hydroperoxide. Synthesis 45(05):615–620. https://doi.org/10.1055/s-0032-1318172
Han X, Wan C, Yang D, Yuan X, Du S, Xiao Y, Qin Z (2013) A highly efficient regioselective addition of acetylides to enediones based on steric effects. Molecules 18(9):10776–10788. https://doi.org/10.3390/molecules180910776
Ertl P, Rohde B, Selzer P (2000) Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem 43(20):3714–3717. https://doi.org/10.1021/jm000942e
Merlot S, Mustilli A-C, Genty B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J 30(5):601–609. https://doi.org/10.1046/j.1365-313X.2002.01322.x
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. https://doi.org/10.1146/annurev.arplant.53.091401.143329
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803. https://doi.org/10.1146/annurev.arplant.57.032905.105444
Acknowledgements
This research was supported financially by the National Natural Science Foundation of China (No. 21572265).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Wan, C., Li, J., Zhao, F. et al. Synthesis and plant growth regulatory activities of 2′,3′-PhABA and iso-2′,3′-PhABA esters. Mol Divers 24, 119–130 (2020). https://doi.org/10.1007/s11030-019-09931-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09931-w