Abstract
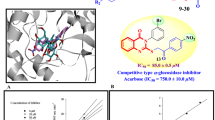
This study is focused on the identification of thiazole-based inhibitors for the \(\alpha \)-glucosidase enzyme. For that purpose, (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives were synthesized in two steps and characterized by various spectroscopic techniques. All derivatives and intermediates were evaluated for their in vitro \(\alpha \)-glucosidase inhibitory activity. Thiosemicarbazones 20 and 35, and cyclized thiazole derivatives 2, 5–11, 13, 15, 21–24, 27–31, and 36–37 showed significant inhibitory potential in the range of \(\hbox {IC}_{50}=6.2\pm 0.19\)–\(43.6\pm 0.23~\upmu \hbox {M}\) as compared to standard acarbose (\(\hbox {IC}_{50}=37.7\pm 0.19~\upmu \hbox {M}\)). A molecular modeling study was carried out to understand the binding interactions of compounds with the active site of enzyme.











Similar content being viewed by others
References
Kasturi S, Surarapu S, Uppalanchi S, Anireddy JS, Dwivedi S, Anantaraju HS, Ethiraj KS (2017) Synthesis and \(\alpha \)-glucosidase inhibition activity of dihydroxy pyrrolidines. Bioorg Med Chem Lett 27:2818–2823. https://doi.org/10.1016/j.bmcl.2017.04.078
Yue LM, Lee J, Zheng L, Park YD, Ye ZM, Yang JM (2017) Computational prediction integrating the inhibition kinetics of gallotannin on \(\alpha \)-glucosidase. Int J Biol Macromol 103:829–838. https://doi.org/10.1016/j.ijbiomac.2017.05.106
Hatano A, Kanno Y, Kondo Y, Sunaga Y, Umezawa H, Okada M, Fukui K (2017) Synthesis and characterization of novel, conjugated, fluorescent DNJ derivatives for \(\alpha \)-glucosidase recognition. Bioorg Med Chem 25:773–778. https://doi.org/10.1016/j.bmc.2016.11.053
Liu DM, Chen J, Shi YP (2017) Screening of enzyme inhibitors from traditional Chinese medicine by magnetic immobilized \(\alpha \)-glucosidase coupled with capillary electrophoresis. Talanta 164:548–555. https://doi.org/10.1016/j.talanta.2016.12.028
Chen H, Ouyang K, Jiang Y, Yang Z, Hu W, Xiong L, Wang W (2017) Constituent analysis of the ethanol extracts of Chimonanthus nitens Oliv. leaves and their inhibitory effect on \(\alpha \)-glucosidase activity. Int J Biol Macromol 98:829–836. https://doi.org/10.1016/j.ijbiomac.2017.02.044
Chaudhry F, Choudhry S, Huma R, Ashraf M, al-Rashida A, Munir R, Khan MA (2017) Hetarylcoumarins: synthesis and biological evaluation as potent \(\alpha \)-glucosidase inhibitors. Bioorg Chem 73:1–9. https://doi.org/10.1016/j.bioorg.2017.05.009
Chaudhry F, Naureen S, Huma R, Shaukat A, al-Rashida M, Asif N, Khan MA (2017) In search of new \(\alpha \)-glucosidase inhibitors: imidazolylpyrazole derivatives. Bioorg Chem 71:102–109. https://doi.org/10.1016/j.bioorg.2017.01.017
Yar M, Bajda M, Shahzad S, Ullah N, Gilani MA, Ashraf M, Shaukat A (2015) Organocatalyzed solvent free an efficient novel synthesis of 2,4,5-trisubstituted imidazoles for \(\alpha \)-glucosidase inhibition to treat diabetes. Bioorg Chem 58:65–71. https://doi.org/10.1016/j.bioorg.2014.11.006
Asano N (2003) Glycosidase inhibitors: update and perspectives on practical use. Glycobiology 13:93R–104R. https://doi.org/10.1093/glycob/cwg090
Toeller M (1994) \(\alpha \)-Glucosidase inhibitors in diabetes: efficacy in NIDDM subjects. Eur J Clin Invest 24:31–35. https://doi.org/10.1111/j.1365-2362.1994.tb02253.x
Scott LJ, Spencer CM (2000) Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs 59:521–549
Ag H (1994) Pharmacology of \(\alpha \)-glucosidase inhibition. Eur J Clin Invest 24:3–10
Khan KM, Qurban S, Salar U, Taha M, Hussain S, Perveen S, Wadood A (2016) Synthesis, in vitro \(\alpha \)-glucosidase inhibitory activity and molecular docking studies of new thiazole derivatives. Bioorg Chem 68:245–258. https://doi.org/10.1016/j.bioorg.2016.08.010
Mouri K, Saito S, Yamaguchi S (2012) Highly flexible \(\pi \)-expanded cyclooctatetraenes: cyclic thiazole tetramers with head-to-tail connection. Angew Chem Int Ed 51:5971–5975. https://doi.org/10.1002/anie.201201265
Frija LM, Pombeiro AJ, Kopylovich MN (2016) Coordination chemistry of thiazoles, isothiazoles and thiadiazoles. Coord Chem Rev 308:32–55. https://doi.org/10.1016/j.ccr.2015.10.003
Mishra CB, Kumari S, Tiwari M (2015) Thiazole: a promising heterocycle for the development of potent CNS active agents. Eur J Med Chem 92:1–34. https://doi.org/10.1016/j.ejmech.2014.12.031
Rouf A, Tanyeli C (2015) Bioactive thiazole and benzothiazole derivatives. Eur J Med Chem 97:911–927. https://doi.org/10.1016/j.ejmech.2014.10.058
Dawood KM, Eldebss TM, El-Zahabi HS, Yousef MH (2015) Synthesis and antiviral activity of some new bis-1,3-thiazole derivatives. Eur J Med Chem 102:266–276. https://doi.org/10.1016/j.ejmech.2015.08.005
Maienfisch P, Edmunds AJ (2017) Chapter three-thiazole and isothiazole ring-containing compounds in crop protection. Adv Heterocycl Chem 121:35–88. https://doi.org/10.1016/bs.aihch.2016.04.010
Salar U, Khan KM, Iqbal J, Ejaz SA, Hameed A, Al-Rashida M, Perveen S, Tahir MN (2017) Coumarin sulfonates: new alkaline phosphatase inhibitors; in vitro and in silico studies. Eur J Med Chem 131:29–47. https://doi.org/10.1016/j.ejmech.2017.03.003
Salar U, Khan KM, Taha M, Ismail NH, Ali B, Qurat-ul-Ain Perveen S, Ghufran M, Wadood A (2017) Biology-oriented drug synthesis (BIODS): in vitro \(\beta \)-glucuronidase inhibitory and in silico studies on 2-(2-methyl-5-nitro-1\(H\)-imidazol-1-yl) ethyl aryl carboxylate derivatives. Eur J Med Chem 125:1289–1299. https://doi.org/10.1016/j.ejmech.2016.11.031
Salar U, Khan KM, Syed S, Taha M, Ali F, Ismail NH, Perveen S, Wadood A, Ghufran M (2017) Synthesis, in vitro \(\beta \)-glucuronidase inhibitory activity and in silico studies of novel \((E)\)-4-Aryl-2-(2-(pyren-1-ylmethylene) hydrazinyl) thiazoles. Bioorg Chem 70:199–209. https://doi.org/10.1016/j.bioorg.2016.12.011
Arshad T, Khan KM, Rasool N, Salar U, Hussain S, Asghar H, Ashraf M, Wadood A, Riaz M, Perveen S, Taha M, Ismail NH (2017) 5-Bromo-2-aryl benzimidazole derivatives as non-cytotoxic potential dual inhibitors of \(\alpha \)-glucosidase and urease enzymes. Bioorg Chem 72:21–31. https://doi.org/10.1016/j.bioorg.2017.03.007
Salar U, Taha M, Ismail NH, Khan KM, Imran S, Perveen S, Wadood A, Riaz M (2016) Thiadiazole derivatives as new class of \(\beta \)-glucuronidase inhibitors. Bioorg Med Chem 24:1909–1918. https://doi.org/10.1016/j.bmc.2016.03.020
Ali F, Khan KM, Salar U, Iqbal S, Taha M, Ismail NH, Perveen S, Wadood A, Ghufran M, Ali B (2016) Dihydropyrimidones: as novel class of \(\beta \)-glucuronidase inhibitors. Bioorg Med Chem 24:3624–3635. https://doi.org/10.1016/j.bmc.2016.06.002
Salar U, Taha M, Khan KM, Ismail NH, Imran S, Perveen S, Gul S, Wadood A (2016) Syntheses of new 3-thiazolyl coumarin derivatives, in vitro \(\alpha \)-glucosidase inhibitory activity, and molecular modeling studies. Eur J Med Chem 122:196–204. https://doi.org/10.1016/j.ejmech.2016.06.037
Ali F, Khan KM, Salar U, Taha M, Ismail NH, Wadood A, Riaz M, Perveen S (2017) Hydrazinyl arylthiazole based pyridine scaffolds: synthesis, structural characterization, in vitro \(\alpha \)-glucosidase inhibitory activity, and in silico studies. Eur J Med Chem 138:255–272. https://doi.org/10.1016/j.ejmech.2017.06.041
Rahim F, Ullah Hayat, Javid MT, Wadood A, Taha M, Ashraf M, Shaukat A, Junaid M, Hussain S, Rehman W, Mehmood R, Sajid M, Khan MN, Khan KM (2015) Synthesis, in vitro evaluation and molecular docking studies of thiazole derivatives as new inhibitors of \(\alpha \)-glucosidase. Bioorg Chem 62:15–21. https://doi.org/10.1016/j.bioorg.2015.06.006
Molecular Operating Environment (MOE), 2016.08; Chemical Computing Group Inc., 1010 Sherbrooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2016
Ferreira SB, Sodero ACR, Cardoso MFC, Lima ES, Kaiser CR, Silva FP, Ferreira VF (2010) Synthesis, biological activity, and molecular modeling studies of 1\(H\)-1,2,3-triazole derivatives of carbohydrates as \(\alpha \)-glucosidases inhibitors. J Med Chem 53:2364–2375. https://doi.org/10.1021/jm901265h
Park JH, Ko S, Park H (2008) Toward the virtual screening of \(\alpha \)-glucosidase inhibitors with the homology-modeled protein structure. Bull Korean Chem Soc 29:921–927. https://doi.org/10.5012/bkcs.2008.29.5.921
Roujeinikova A, Raasch C, Sedelnikova S, Liebl W, Rice W (2002) Crystal structure of Thermotoga maritima 4-\(\alpha \)-glucanotransferase and its acarbose complex: implications for substrate specificity and catalysis. J Mol Biol 321:149–162. https://doi.org/10.1016/S0022-2836(02)00570-3
Guerreiro LR, Carriero EP, Fernandes L, Cardote TA, Moreira R, Caldeira AT, Guedes RC, Burke AJ (2013) Five-membered iminocyclitol \(\alpha \)-glucosidase inhibitors: synthetic, biological screening and in silico studies. Bioorg Med Chem 21:1911–1917. https://doi.org/10.1016/j.bmc.2013.01.030
Taha M, Ismail NH, Imran S, Wadood A, Ali M, Rahim F, Khan AA, Riaz M (2016) Novel thiosemicarbazide-oxadiazole hybrids as unprecedented inhibitors of yeast \(\alpha \)-glucosidase and in silico binding analysis. RSC Adv 6:33733–33742. https://doi.org/10.1039/C5RA28012E
Turner S, Myers M, Gadie B, Nelson AJ, Pape R, Saville JF, Doxey JC, Berridge TL (1988) Antihypertensive thiadiazoles. 1. Synthesis of some 2-aryl-5-hydrazino-1,3,4-thiadiazoles with vasodilator activity. J Med Chem 31:902–906. https://doi.org/10.1021/jm00400a003
Osman H, Arshad A, Lam CK, Bagley MC (2012) Microwave-assisted synthesis and antioxidant properties of hydrazinyl thiazolyl coumarin derivatives. Chem Cent J 6:32. https://doi.org/10.1186/1752-153X-6-32
Hassan AA, Mohamed SK, Mohamed NK, El-Shaieb KM, Abdel-Aziz AT, Mague JT, Akkurt M (2016) Synthesis of 1,3-thiazolidin-4-ones; reactivity of the thiosemicarbazone function towards dimethyl acetylenedicarboxylate. J Chem Res 40:173–177. https://doi.org/10.3184/174751916X14552868764580
Subhashree GR, Haribabu J, Saranya S, Yuvaraj P, Anantha Krishnan D, Karvembu R, Gayathri D (2017) In vitro antioxidant, antiinflammatory and in silico molecular docking studies of thiosemicarbazones. J Mol Str 1145:160–169. https://doi.org/10.1016/j.molstruc.2017.05.054
Linciano P, Moraes CB, Alcantara LM, Franco CH, Pascoalino B, Freitas-Junior LH, Macedo S, Santarem N, Cordeiro-da-Silva A, Gul S, Witt G, Kuzikove M, Ellingere B, Ferraria S, Luciania R, Quotadamoa A, Costantinoa L, Costia MP (2018) Aryl thiosemicarbazones for the treatment of trypanosomatidic infections. Eur J Med Chem 146:423–434. https://doi.org/10.1016/j.ejmech.2018.01.043
Alagramam KN, Gopal SR, Geng R, Chen DH, Nemet I, Lee R, Tian G, Miyagi M, Malagu KF, Lock CJ, Esmieu WR (2016) A small molecule mitigates hearing loss in a mouse model of Usher syndrome III. Nat Chem Bio 12:444–451. https://doi.org/10.1038/nchembio.2069
Chimenti F, Bizzarri B, Bolasco A, Secci D, Chimenti P, Granese A, Carradori S, D’Ascenzio M, Lilli D, Rivanera D (2011) Synthesis and biological evaluation of novel 2, 4-disubstituted-1,3-thiazoles as anti-Candida spp. agents. Eur J Med Chem 46:378–382. https://doi.org/10.1016/j.ejmech.2010.10.027
Abdelhamid AO, Ismail ZH, El Gendy MS, Ghorab MM (2007) Reactions of hydrazonoyl halides \(54^{1}\): synthesis and reactivity of 3-aza-2-bromo-1-(3-oxo benzo[\(f\)]chromen-2-yl-3-(arylamino)prop-2-en-1-one. Phosphorus Sulfur Silicon Relat Elem 182:2409–2418. https://doi.org/10.1080/10426500701521548
Cai CY, Rao L, Rao Y, Guo JX, Xiao ZZ, Cao JY, Huang ZS, Wang B (2017) Analogues of xanthones-chalcones and bis-chalcones as \(\alpha \)-glucosidase inhibitors and anti-diabetes candidates. Eur J Med Chem 130:51–59. https://doi.org/10.1016/j.ejmech.2017.02.007
Acknowledgements
This work was supported by the Higher Education Commission (HEC) Pakistan under the National Research Program for Universities (Project No. 20-1910).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, M., Khan, K.M., Salar, U. et al. Synthesis, in vitro \(\alpha \)-glucosidase inhibitory activity, and in silico study of (E)-thiosemicarbazones and (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives. Mol Divers 22, 841–861 (2018). https://doi.org/10.1007/s11030-018-9835-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9835-2