Abstract
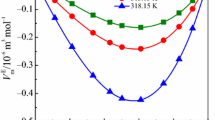
Density (ρ) and speed of sound (u) of binary liquid mixtures of dimethyl carbonate and N-methylformamide have been determined at T = (303.15, 308.15, 313.15 and 318.15) K over the entire composition range. Experimental data are used to evaluate excess values of molar volume (\(V_{\text{m}}^{\text{E}}\)), isentropic compressibility (\(k_{\text{s}}^{\text{E}}\)), isothermal compressibility (\(k_{\text{T}}^{\text{E}}\)), intermolecular free length (\(L_{\text{f}}^{\text{E}}\)), acoustic impedance (\(Z^{\text{E}}\)) and ultrasonic speed (\(u^{\text{E}}\)). The VE data in the present investigation were analysed by using Prigogine–Flory–Patterson (PFP) theory. Partial and excess partial molar volumes (\(\bar{V}_{{{\text{m}},1}}\), \(\bar{V}_{{{\text{m}},2}}\)), (\(\bar{V}_{{{\text{m}},1}}^{\text{E}}\), \(\bar{V}_{{{\text{m}},2}}^{\text{E}}\)) and partial and excess partial molar volume of the components at infinite dilution (\(\overline{V}_{{{\text{m}},1}}^{\infty }\), \(\overline{V}_{{{\text{m}},2}}^{\infty }\)), (\(\overline{V}_{{{\text{m}},1}}^{{{\text{E}},\infty }}\), \(\overline{V}_{{{\text{m}},2}}^{{{\text{E}},\infty }}\)) at T = (303.15, 308.15, 313.15, 318.15) K have been calculated. The excess/deviation properties were fitted to Redlich–Kister equation to obtain their coefficients and standard deviations. The present investigation also comprises the acoustic nonlinearity parameter (B/A) in the mixtures and calculation of cohesive energy \(\Delta A\), Van der Wall’s constants (a, b) and distance of closest approach (d). Moreover, various semi-empirical relations of ultrasonic speed have been used to correlate the theoretical velocities. FT-IR spectra of pure components and their binaries have been measured at T = 298.15 K.







Similar content being viewed by others
References
Satyanarayana GR, Bala Karuna Kumar D, Sujatha K, Lakshmanarao G, Rambabu C. Probing the intermolecular interactions in the binary liquid mixtures of o-chlorophenol with alkoxyethanols through ultrasonic, transport and FT-IR spectroscopic studies at different temperatures. J Mol Liq. 2016;216:526–37.
Moreiras AF, Garcia J, Lugo L, Comunas MJP, Lopez ER, Fernandez J. Experimental densities and dynamic viscosities of organic carbonate + n-alkane or p-xylene systems at 298.15 K. Fluid Phase Equilib. 2003;204:233–43.
Zafarani-Moattar MT, Izadi F. Effect of KCl on the volumetric and transport properties of aqueous tri-potassium citrate solutions at different temperatures. J Chem Thermodyn. 2011;43:552–61.
Kannappan V, Hemalatha G. Ultrasonic studies on the molecular interaction of 1-chlorobenzotriazole with aromatic compounds in solution. Indian J Pure Appl Phys. 2005;43:849–53.
Zorebski E, Kostka BL. Thermodynamic and transport properties of (1, 2-ethanediol +1- nonanol) at temperatures (298.15 to 313.15) K. J Chem Thermodyn. 2008;41:197–204.
Syamala V, Venkatramana L, Narasimha Rao C, Sivakumar K, Venkateswarlu P, Gardas RL. Effect of various substituents on benzene ring and their impact on volumetric, acoustic and transport properties of binary liquid mixtures with dimethylacetamide. Fluid Phase Equilib. 2015;397:68–80.
Romanoa E, Trenzado JL, Gonzalez E, Matos JS, Segade L, Jimenez E. Thermophysical properties of four binary dimethyl carbonate + 1-alcohol systems at 288.15-313.15 K. Fluid Phase Equilib. 2003;211:219–40.
Tobishima SI, Arakawa M, Yamaki JI. Electrolytic properties of LiClO4—propylene carbonate mixed with amide-solvents for lithium batteries. Electrochim Acta. 1988;33:239–44.
Pacheco MA, Marshall CL. Review of dimethyl carbonate (DMC) manufacture and its characteristics as a fuel additive. Energy Fuels. 1997;11:2–29.
Fang YJ, Qian JM. Isobaric vapor–liquid equilibria of binary mixtures containing the carbonate group −OCOO−. J Chem Eng Data. 2005;50:340–3.
Sharma VK, Rajni D, Sharma D. Excess molar volumes and excess isentropic compressibilities of binary and ternary mixtures of o-chlorotoluene with cyclic ether and amides or cyclohexane at different temperatures. J Chem Thermodyn. 2014;78:241–53.
Lugo L, Comunas MJP, Lopez ER, Fernandez J. (p, V m, T, x) measurements of dimethyl carbonate + octane binary mixtures: I. Experimental results, isothermal compressibilities, isobaric expansivities and internal pressures. Fluid Phase Equilib. 2001;186:235–55.
Shin SH, Jeong IY, Jeong YS, Park SJ. Solid–liquid equilibria and the physical properties of binary systems of diphenyl carbonate, dimethyl carbonate, methyl phenyl carbonate, anisole, methanol and phenol. Fluid Phase Equilib. 2014;376:105–10.
Iglesias-Otero MA, Troncoso J, Carballo E, Romani L. Density and refractive index for binary systems of the ionic liquid [Bmim][BF4] with methanol, 1,3-dichloropropane, and dimethyl carbonate. J Solution Chem. 2007;36:1219–30.
Chen F, Yang Z, Chen Z, Hu J, Chen C, Cai J. Density, viscosity, speed of sound, excess property and bulk modulus of binary mixtures of γ-butyrolactone with acetonitrile, dimethyl carbonate, and tetrahydrofuran at temperatures (293.15 to 333.15) K. J Mol Liq. 2015;209:683–92.
Riddick JA, Bunger WB, Sakano TK. Organic Solvents, Physical properties and methods of purification. 4th ed. New York: Wiley-Interscience; 1986.
Ren R, Zuo Y, Zhou Q, Zhang H, Zhang S. Density, excess molar volume and conductivity of binary mixtures of the ionic liquid 1,2-dimethyl-3-hexylimidazolium bis(trifluoromethylsulfonyl)imide and dimethyl carbonate. J Chem Eng Data. 2011;56:27–30.
Rodriguez A, Canosa J, Tojo J. Physical properties of binary mixtures (dimethyl carbonate +alcohols) at several temperatures. J Chem Eng Data. 2001;46:1476–86.
Pardo JM, Gonzalez-Salgado D, Tovar CA, Cerdeirina CA, Carballo E, Romani L. Comparative study of the thermodynamic behaviour of the binary mixtures dimethyl carbonate + (benzene, n-heptane, cyclohexane, or toluene). Can J Chem. 2002;80:370–8.
Pardo JM, Tovar CA, Cerdeirina CA, Carballo E, Romani L. Excess quantities of dialkyl carbonate + cyclohexane mixtures at a variable temperature. Fluid Phase Equilib. 2001;179:151–63.
Vranes M, Tot A, Zec N, Papovic S, Gadzuric S. Volumetric properties of binary mixtures of 1-butyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate with N-methylformamide, N-ethylformamide, N,N-dimethylformamide, N,N-dibutylformamide, and N,N-dimethylacetamide from (293.15 to 323.15) K. J Chem Eng Data. 2014;59:3372–9.
Balaji R, Gowri Sankar M, Chandra Sekhar M, Chandra Shekar M. Thermodynamic properties of n-methylformamide + short carboxylic acids as a function of temperature. Karbala Int J Modern Sci. 2016;2:10–9.
Sharma VK, Bhagour S. Molecular interactions in 1-ethyl-3-methylimidazolim tetrafluoroborate + amide mixtures: excess molar volumes, excess isentropic compressibilities and excess molar enthalpies. J Solution Chem. 2013;42:800–22.
Rowlinson JS, Swinton FL. Liquid and liquid mixtures. 3rd ed. London: Butterworths; 1982. p. 16–7.
Hirschfelder JO, Curtis CF, Bird RB. Molecular theory of gases and liquids. New York: Wiley; 1964 (corrected printing).
Jyothirmai G, Nayeem SM, Khan I, Anjaneyulu C. Thermo-physicochemical investigation of molecular interactions in binary combination (dimethyl carbonate + methyl benzoate). J Therm Anal Calorim. 2018;132:693–707.
Benson GC, Kiyohara OJ. Thermodynamic properties of some cycloalkane-cycloalkanol systems at 298.15 K. Chem Eng Data. 1976;21:362–5.
Redlich O, Kister AT. Thermodynamic of non electrolyte solutions: algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Bakshi MS, Singh J, Kaur H, Ahmad ST, Kaur G. Thermodynamic behaviour of mixtures part-3 mixtures of acetonitrile with dimethylacetamide, dimethylsulphoxide, nitrobenzene and methanol at 25°C. J Chem Eng Data. 1996;41:1459–61.
Garcia B, Alcalde R, Leal JM, Matos JS. Formamide–(C1–C5) alkan-1-ols solvent systems. J Chem Soc, Faraday Trans. 1996;92:3347–52.
Sankar MG, Ponneri V, Kumar KS, Sakamuri S. Molecular interactions between amine and cyclic ketones at different temperatures. J Therm Anal Calorim. 2014;115:1821–7.
Nain AK. Densities, ultrasonic speeds, viscosities and excess properties of binary mixtures of methyl methacrylate with N,N-dimethylformamide and N,N-dimethylacetamide at different temperatures. J Chem Thermodyn. 2013;60:105–16.
Kondaiah M, Sreekanth K, Sravana Kumar D, Nayeem SM, Krishna Rao D. Densities, viscosities, and excess properties for binary mixtures of ethylene glycol with amides at 308.15 K. J Therm Anal Calorim. 2014;118:475–83.
Rajagopal K, Chenthilnath S. Excess thermodynamic studies of binary liquid mixtures of 2-methyl-2-propanol with ketones. Indian J Pure Appl Phys. 2010;48:326–33.
Nayeem SM, Kondaiah M, Sreekanth K, Krishna Rao D. Acoustic and volumetric investigations in aromatic, cyclic and aliphatic ketones with dimethyl sulphoxide at 308.15 K. Arab J Chem. 2015. https://doi.org/10.1016/j.arabjc.2015.08.005.
Nam-Tran HA. New model for evaluating interactions in liquids. J Phys Chem. 1994;98:5362–7.
Balaji R, Gowri Sankar M, Chandra Sekhar M, Chandra Shekar M. Thermophysical and spectroscopic properties of binary liquid systems: acetophenone/cyclopentanone/cyclohexanone with N-methylformamide. Phys Chem Liquids. 2015. https://doi.org/10.1080/00319104.2015.1109996.
Aroni F, Kelarakis A, Havredaki V. Volumetric behavior of a bolaamphiphile in different amides–water and ethylene glycol–water mixtures. J Colloid Interface Sci. 2005;292:236–43.
Umadevi P, Rambabu K, Rao MN, Rao KS, Rambabu C. Densities, adiabatic compressibility, free-length, viscosities and excess volumes of P-cresol(1) + dimethyl sulfoxide (2), + dimethyl formamide (2), and + 1,4-dioxane at 303.15–318.15 K. Phys Chem Liq. 1995;30:29–46.
Ali A, Nain AK. Ultrsonic and volumetric study of binary mixtures of benzyl alcohol with amides. Bull Chem Soc Jpn. 2002;75:681–7.
Venkatramana L, Sreenivasulu K, Sivakumar K, Reddy KD. Thermodynamic properties of binary mixtures containing 1-alkanols. J Therm Anal Calorim. 2014;115:1829–34.
Rastogi M, Awasthi A, Gupta M, Shukla JP. Ultrasonic investigations of X… HO bond complexes. Indian J Pure Appl Phys. 2002;40:256–63.
Baragi JG, Mutalik VK, Mekali SB. Molecular interaction studies in mixtures of methylcyclohexane with alkanes: a theoretical approach. Int J Pharm Bio Sci. 2013;3:185–97.
Nain AK. Densities and volumetric properties of (acetonitrile + an amide) binary mixtures at temperatures between 293.15 K and 318.15 K. J Chem Thermodyn. 2006;38:1362–70.
Flory PJ. Statistical thermodynamics of liquid mixtures. J Am Chem Soc. 1965;87:1833–8.
Abe A, Flory PJ. The thermodynamic properties of mixtures of small, nonpolar molecules. J Am Chem Soc. 1965;87:1838–46.
Iloukhani H, Almasi M. Densities and excess molar volumes of binary and ternary mixtures containing acetonitrile + acetophenone + 1,2-pentanediol: experimental data, correlation and prediction by PFP theory and ERAS model. J Solution Chem. 2011;40:284–98.
Letcher TM, Baxter RC. Application of the Prigogine–Flory–Patterson theory part III. Mixtures of a bicyclic compound, benzene, cyclohexane, n-hexane with a cycloalkane, cyclohexene, a cycloalkadiene and benzene. J Solution Chem. 1989;18:89–97.
Fort RJ, Moore WR. Adiabatic compressibilities of binary liquid mixtures. J Trans Faraday Soc. 1965;61:2102–11.
Comelli F, Ottani S, Francesconi R, Castellari C. Densities, viscosities, and refractive indices of binary mixtures containing n-hexane + components of pine resins and essential oils at 298.15 K. J Chem Eng Data. 2002;47:93–7.
Ali A, Nabi F, Tariq M. Volumetric, viscometric, ultrasonic, and refractive index properties of liquid mixtures of benzene with industrially important monomers at different temperatures. Int J Thermophys. 2009;30:464–74.
Prakash Dubey G, Kumar K. Studies of thermodynamic, thermophysical and partial molar properties of liquid mixtures of diethylenetriamine with alcohols at 293.15 to 313.15 K. J Mol Liq. 2013;180:164–71.
Govardhana Rao S, Madhu Mohan T, Vijaya Krishna T, Srinivasa Krishna T, Subba Rao B. Density, refractive index, and speed of sound of the binary mixture of 1-butyl-3-methylimidazolium tetrafluoroborate + n-vinyl-2-pyrrolidinone from t = (298.15 to 323.15) K at atmospheric pressure. J Chem Eng Data. 2015;60:886–94.
Bhalodia J, Sharma S. Volumetric, refractive and FT-IR behaviour of β-pinene with o, m, p-cresol at 303.15, 308.15 and 313.15 K. J Mol Liq. 2014;193:249–55.
Wisniak J, Cortez G, Peralta RD, Infante R, Elizalde LE, Amaro TA, Garcia O, Soto H. Density, excess volume, and excess coefficient of thermal expansion of the binary systems of dimethyl carbonate with butyl methacrylate, allyl methacrylate, styrene, and vinyl acetate at T = (293.15, 303.15, 313.15) K. J Chem Thermodyn. 2008;40:1671–83.
Acknowledgements
One of the authors, Sk. Beebi, wishes to thank the Department of Chemistry and Department of Physics, Acharya Nagarjuna University, Nagarjuna Nagar, Guntur, for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beebi, S., Nayeem, S.M. & Rambabu, C. Investigation of molecular interactions in binary mixture of dimethyl carbonate + N-methylformamide at T = (303.15, 308.15, 313.15 and 318.15) K. J Therm Anal Calorim 135, 3387–3399 (2019). https://doi.org/10.1007/s10973-018-7574-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7574-3