Abstract
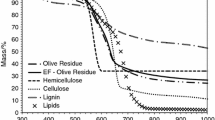
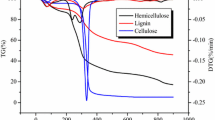
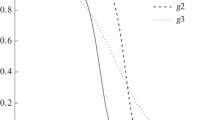
This paper discusses the different methods for determining the kinetic parameters (activation energy and pre-exponential factor) of biomass (beechwood and flax shives) pyrolysis based on Kissinger method, isoconversional methods (Kissinger–Akahira–Sunose and Friedman) and based model (nonlinear least square minimization and optimization by genetic algorithm). Because of the widely dispersed values of activation energy and pre-exponential factor of three pseudo-components of the biomass (cellulose, hemicellulose and lignin) found in the literature, the pyrolysis of cellulose, hemicellulose and lignin was also studied. This paper shows that kinetic parameters are very sensitive to methods used. The comparison of results shows a large difference for the same experimental results even for pure pseudo-components. Based on results comparison, we think that Kissinger method remains the best method for kinetic parameters determination. Indeed, Kissinger relation takes into account the biomass structure effect and the mineral content. Isoconversional methods are also very suitable for low and medium conversion rate. Despite the fact that based methods are considered to be robust methods for the estimation of kinetic parameters in chemical engineering, these methods may misestimate the activation energy and pre-exponential factor for cellulose, hemicellulose and lignin.







Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor [s−1]
- A α :
-
Pre-exponential factor at a given conversion degree [s−1]
- E a :
-
Activation energy [kJ mol−1]
- E α :
-
Activation energy at a given conversion degree [kJ mol−1]
- GA:
-
Genetic algorithm
- KAS:
-
Kissinger–Akahira–Sunose
- m f :
-
Final mass [kg]
- m i :
-
Initial mass [kg]
- m T :
-
Mass at temperature T [kg]
- N :
-
Number of experiments considered
- n :
-
Reaction order [−]
- NLSM:
-
Nonlinear least squares optimization method
- OFW:
-
Ozawa, Flynn and wall
- R :
-
Gas constant [8.314 J K−1 mol−1]
- R²:
-
Correlation coefficient
- S :
-
Convergence criterion [s-2]
- T :
-
Temperature [K]
- TGA:
-
Thermogravimetric analysis
- T max :
-
Maximum temperature peak [K]
- T offset :
-
Represents the end of the decomposition determined by the extrapolation of the DTG curves
- T onset :
-
The temperature of start of the decomposition
- α :
-
Conversion degree [−]
- β :
-
Heating rate [K s−1]
References
Zhao H, Yan H, Zhang C, Sun B, Zhang Y, Dong S, Xue Y, Qin S. Thermogravimetry study of pyrolytic characteristics and kinetics of the giant wetland plant Phragmites australis. J Therm Anal Calorim. 2012;110:611–7.
Blasi CD, Russo G. Modeling of transport phenomena and kinetics of biomass pyrolysis. 1993. doi:10.1007/978-94-011-1336-6_70.
Sanchez-Silva L, López-González D, Villaseñor J, Sánchez P, Valverde JL. Thermogravimetric–mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour Technol. 2012;109:163–72.
Jiang G, Nowakowski DJ, Bridgwater AV. A systematic study of the kinetics of lignin pyrolysis. Thermochim Acta. 2010;498:61–6.
Grønli MG, Varhegyi G, Di Blasi C. Thermogravimetric analysis and devolatilization kinetics of wood. Ind Eng Chem Res. 2002;41:4201–8.
Anca-Couce A, Berger A, Zobel N. How to determine consistent biomass pyrolysis kinetics in a parallel reaction scheme. Fuel. 2014;123:230–40.
Chen Z, Zhu Q, Wang X, Xiao B, Liu S. Pyrolysis behaviors and kinetic studies on Eucalyptus residues using thermogravimetric analysis. Energy Convers Manag. 2015;105:251–9.
Grønli M, Antal MJ, Várhegyi G. A round-robin study of cellulose pyrolysis kinetics by thermogravimetry. Ind Eng Chem Res. 1999;38:2238–44.
Di Blasi C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog Energy Combust Sci. 2008;34:47–90.
Sonoyama N, Hayashi J. Characterisation of coal and biomass based on kinetic parameter distributions for pyrolysis. Fuel. 2013;114:206–15.
Stenseng M, Jensen A, Dam-Johansen K. Investigation of biomass pyrolysis by thermogravimetric analysis and differential scanning calorimetry. J Anal Appl Pyrolysis. 2001;58:765–80.
Vyazovkin S, Dollimore D. Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids. J Chem Inf Comput Sci. 1996;36:42–5.
Wu W, Cai J, Liu R. Isoconversional kinetic analysis of distributed activation energy model processes for pyrolysis of solid fuels. Ind Eng Chem Res. 2013;52:14376–83.
de Baroni É, Tannous K, Rueda-Ordóñez YJ, Tinoco-Navarro LK. The applicability of isoconversional models in estimating the kinetic parameters of biomass pyrolysis. J Therm Anal Calorim. 2016;123:909–17.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Slopiecka K, Bartocci P, Fantozzi F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl Energy. 2012;97:491–7.
Khawam A, Flanagan DR. Complementary use of model-free and modelistic methods in the analysis of solid-state kinetics. J Phys Chem B. 2005;109:10073–80.
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour Technol. 2011;102:6230–8.
Magdziarz A, Wilk M, Straka R. Combustion process of torrefied wood biomass. J Therm Anal Calorim 2016. doi:10.1007/s10973-016-5731-0.
Nowak B, Karlström O, Backman P, Brink A, Zevenhoven M, Voglsam S, Winter F, Hupa M. Mass transfer limitation in thermogravimetry of biomass gasification. J Therm Anal Calorim. 2012;111:183–92.
Soria-Verdugo A, Goos E, García-Hernando N. Effect of the number of TGA curves employed on the biomass pyrolysis kinetics results obtained using the distributed activation energy model. Fuel Process Technol. 2015;134:360–71.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340:53–68.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Cai J, Alimujiang S. Kinetic analysis of wheat straw oxidative pyrolysis using thermogravimetric analysis: statistical description and isoconversional kinetic analysis. Ind Eng Chem Res. 2008;48:619–24.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature 1964;201:68–9.
Soria-Verdugo A, García-Hernando N, Garcia-Gutierrez LM, Ruiz-Rivas U. Analysis of biomass and sewage sludge devolatilization using the distributed activation energy model. Energy Convers Manag. 2013;65:239–44.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92.
Balland L, Estel L, Cosmao J-M, Mouhab N. A genetic algorithm with decimal coding for the estimation of kinetic and energetic parameters. Chemom Intell Lab Syst. 2000;50:121–35.
Balland L, Mouhab N, Cosmao J-M, Estel L. Kinetic parameter estimation of solvent-free reactions: application to esterification of acetic anhydride by methanol. Chem Eng Process Process Intensif. 2002;41:395–402.
Hu Z, Chen Z, Li G, Chen X, Hu M, Laghari M, Wang X, Guo D. Characteristics and kinetic studies of Hydrilla verticillata pyrolysis via thermogravimetric analysis. Bioresour Technol. 2015;194:364–72.
Bhavanam A, Sastry RC. Kinetic study of solid waste pyrolysis using distributed activation energy model. Bioresour Technol. 2015;178:126–31.
Claudy P. Analyse calorimétrique différentielle: théorie et applications de la DSC Lavoisier. Paris; 2005.
Miura K, Maki T. A simple method for estimating f (E) and k 0 (E) in the distributed activation energy model. Energy Fuels. 1998;12:864–9.
White JE, Catallo WJ, Legendre BL. Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. J Anal Appl Pyrolysis. 2011;91:1–33.
Carrier M, Loppinet-Serani A, Denux D, Lasnier J-M, Ham-Pichavant F, Cansell F, Aymonier C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy. 2011;35:298–307.
Cai J, Wu W, Liu R, Huber GW. A distributed activation energy model for the pyrolysis of lignocellulosic biomass. Green Chem. 2013;15:1331–40.
Chen Z, Hu M, Zhu X, Guo D, Liu S, Hu Z, Xiao B, Wang J, Laghari M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour Technol. 2015;192:441–50.
Li Z, Zhao W, Meng B, Liu C, Zhu Q, Zhao G. Kinetic study of corn straw pyrolysis: comparison of two different three-pseudocomponent models. Bioresour Technol. 2008;99:7616–22.
Acknowledgements
This work was supported by the European Union with the European regional development fund (ERDF) and by the Normandie Regional Council.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdelouahed, L., Leveneur, S., Vernieres-Hassimi, L. et al. Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J Therm Anal Calorim 129, 1201–1213 (2017). https://doi.org/10.1007/s10973-017-6212-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6212-9