Abstract
Purpose
Approximately 40% of infertile men have an abnormal semen analysis, resulting from either abnormalities of sperm production (defective spermatogenesis) or sperm shape (defective spermiogenesis). This latter process is dependent upon the function of Sertoli cells, which maintain specialized junctional complexes with germ cells. Nectins, members of the immunoglobulin superfamily, participate in formation of these dynamic complexes. Male mice in which the nectin-2 or nectin-3 gene is knocked out are sterile. Their spermatozoa exhibit severe teratospermia, altered motility, and an impaired ability to fertilize eggs. We asked whether mutations in the protein coding regions of the nectin-2 (aka PVRL2) and nectin-3 (aka PVRL3) genes could be detected in men from infertile couples whose semen analysis revealed unimpaired sperm production, judged by normal sperm concentration, but severe abnormalities in sperm shape.
Methods
Ejaculates were snap frozen in liquid nitrogen and later submitted for Sanger analysis of these two genes, to detect mutations in their protein coding regions.
Results
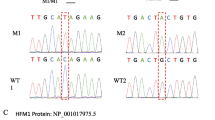
Eighty-nine of 455 ejaculates (19.5%) met the inclusion criteria for study. Two of the 56 samples that were successfully analyzed for nectin-2 (3.6%) and one of 73 (1.3%) analyzed for nectin-3 possessed possibly damaging mutations.
Conclusions
Despite the small-scale nature of the study, at least two low-frequency deleterious variants were identified. These results suggest the need for a larger-scale study of sequence variants in the nectins in severe teratospermia.


Similar content being viewed by others
References
Thonneau P, Marchand S, Tallec A, Ferial M-L, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod. 1991;6:811–6.
Vogl AW, Vaid KS. Guttman JA. The Sertoli cell cytoskeleton. Adv In Exp Med and Biol 2009; 636: 186 - 211.
Marmar JL. The pathophysiology of varicocoeles in the light of current molecular and genetic information. Hum Reprod Update. 2001;7:461–72.
Carlson E, Andersson AM, Petersen JH, Skakkebaek N. History of febrile illness and variation in semen quality. Hum Reprod. 2003;18:2089–92.
Vine MF, Tse CK, Hu P, Truong KY. Cigarette smoking and semen quality. Fertil Steril. 1996;65:835–42.
Kruger TP, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Veeck LL, et al. New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology. 1987;30:246–51.
Bronson RA, Bronson SK, Oula LD. Ability of abnormally-shaped human spermatozoa to adhere to and penetrate zona-free hamster eggs: correlation with sperm morphology and post incubation motility. J Androl. 2007;28:698–705.
Samanta D, Ramagopal UA, Rubinstein R, Vigdorovich V, Nathenson SG, Almo SC. Structure of Nectin-2 reveals determinants of hemophilic and heterothallic interactions that control cell-cell adhesion. PNAS. 2010;109:14836–40.
Mueller S, Rosenquist TA, Takai T, Bronson RA, Wimmer E. Loss of nectin-2 at Sertoli-spermatid junctions leads to male fertility and correlates with severe spermatozoon head and midpiece malformation, impaired binding to the zona pellucida and oocyte penetration. Biol Reprod. 2003;69:1330–40.
Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes to Cells. 2006;11:1123–32.
World Health Organization WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. 4th Ed. Cambridge, United Kingdom, University Press, 1999, 17–22.
Redman JB, Thomas W, Ma W, Drobnes EZ, Sparks A, Wang C, et al. Semen parameters in fertile US men: the study for future families. Andrology. 2013;1(6):806–14.
Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols: methods in molecular biology. Totowa: Humana Press; 2000. p. 365–86.
Adzhube I, Jordan, DM, Sunyaev, SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013; 0 7: Unit7.20. doi:10.1002/0471142905.hg0720s76.
Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Cur Top Dev Biol. 2005;71:263–96.
Massan A, Lissens W, Tournaye H, Stouffs K. Genetic causes of spermatogenic failure. Asian J Andrology. 2014;14:40–8.
Ravel C, Chantot-Bastaraud S, El Houate B, Rouba H, Legendre M, Lorencxo D, et al. Y-chromosome AZFc structural architecture and relationship to male fertility. Fertil Steril. 2009;92:1924–33.
Bashamboo A, Ferraz-Souza B, Lourenco D, Seibire NJ, Montjean D, Bignon-Topalovic J, et al. Human male infertility associated with mutations in NS5A1 encoding steroidogenic factor 1. Am J Human Genet. 2002;87:5005–512.
Imken L, Rouba H, El Houate B, Louanjili N, Barakat A, Chafik A, et al. Mutations in the protamine locus: association with spermatogenic failure. Molec Hum Reprod. 2009;15:733–8.
Montjean D, Zini A, Ravel C, Belloc S, Dalleac A, Copin H, et al. Sperm global DNA methylation level: association with semen parameters and genome integrity. Andrology. 2015;3:235–40.
Eloualid A, Abidi O, Charif M, El Houate B, Beenrahma H, Louanjli N, et al. Association of the MTHFR A1298C variant with unexplained severe male infertility. PLoS One. 2012;7:e3411.
De Boer P, de Vries M, Ramos L. A mutation study of sperm head shape and motility in the mouse: lessons for the clinic. Andrology. 2015;3:174–202.
Koscinski I, Elinati E, Fossard C, Rodin C, Muller J, Barratt CLR, et al. DPY19L2 deletion as a major cause of globozoospermia. Am J Hum Gen. 2011;88:344–50.
Zhu F, Gong F, Lin G, Lu G. DPY19L2 gene mutations are a major cause of globospermia: identification of three novel point mutations. Mol Hum Reprod. 2013;19:395–404.
Pierre V, Martinez G, Coutton C, Delaroche J, Yassine S, Novella C, et al. Absence of Dpy1912, a new inner nuclear membrane protein, causes globospermia in mice by preventing the anchoring of the acrosome to the nucleus. Development. 2012;195:673–87.
Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratospermia: spotlight on the main genetic actors in the human. Hum Reprod Update. 2015;21:455–85.
Acknowledgements
We acknowledge the skillful work of Susan Bronson and Lucila Oula in the Andrology Lab.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Stony Brook University Institutional Review Board [CORIHS No.177570-4], and informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bronson, R., Mikhailik, A., Schwedes, J. et al. Detection of candidate nectin gene mutations in infertile men with severe teratospermia. J Assist Reprod Genet 34, 1295–1302 (2017). https://doi.org/10.1007/s10815-017-0985-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0985-4