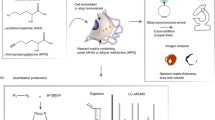
Abstract
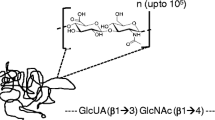
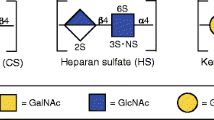
The extracellular matrix (ECM) is a highly dynamic and complex meshwork of proteins and glycosaminoglycans (GAGs) with a crucial role in tissue homeostasis and organization not only by defining tissue architecture and mechanical properties, but also by providing chemical cues that regulate major biological processes. GAGs are associated with important physiological functions, acting as modulators of signaling pathways regulating several cellular processes such as cell growth and differentiation. Recently, in vitro fabricated cell-derived ECM have emerged as promising materials for regenerative medicine due to their ability of better recapitulate the native ECM-like composition and structure, without the limitations of availability and pathogen transfer risks of tissue-derived ECM scaffolds. However, little is known about the molecular and more specifically, GAG composition of these cell-derived ECM. In this study, three different cell-derived ECM were produced in vitro and characterized in terms of their GAG content, composition and sulfation patterns using a highly sensitive liquid chromatography-tandem mass spectrometry technique. Distinct GAG compositions and disaccharide sulfation patterns were verified for the different cell-derived ECM. Additionally, the effect of decellularization method on the GAG and disaccharide relative composition was also assessed. In summary, the method presented here offers a novel approach to determine the GAG composition of cell-derived ECM, which we believe is critical for a better understanding of ECM role in directing cellular responses and has the potential for generating important knowledge to use in the development of novel ECM-like biomaterials for tissue engineering applications.








Similar content being viewed by others
References
Lu, H., Hoshiba, T., Kawazoe, N., Koda, I., Song, M., Chen, G.: Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials. 32, 9658–9666 (2011). https://doi.org/10.1016/j.biomaterials.2011.08.091
Naba, A., Clauser, K.R., Ding, H., Whittaker, C.A., Carr, S.A., Hynes, R.O.: The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. 49, 10–24 (2016). https://doi.org/10.1016/j.matbio.2015.06.003
Bonnans, C., Chou, J., Werb, Z.: Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014). https://doi.org/10.1038/nrm3904
Gilbert, T.W., Sellaro, T.L., Badylak, S.F.: Decellularization of tissues and organs. Biomaterials. 27, 3675–3683 (2006). https://doi.org/10.1016/j.biomaterials.2006.02.014
Badylak, S.F., Taylor, D., Uygun, K.: Whole organ tissue engineering: Decellularization and Recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2010). https://doi.org/10.1146/annurev-bioeng-071910-124743
Wong, M.L., Griffiths, L.G.: Immunogenicity in xenogeneic scaffold generation: Antigen removal vs. decellularization. Acta Biomater. 10, 1806–1816 (2014). https://doi.org/10.1016/j.actbio.2014.01.028
Hoshiba, T., Lu, H., Kawazoe, N., Chen, G.: Decellularized matrices for tissue engineering. Expert. Opin. Biol. Ther. 10, 1717–1728 (2010). https://doi.org/10.1517/14712598.2010.534079
Lu, H., Hoshiba, T., Kawazoe, N., Chen, G.: Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials. 32, 2489–2499 (2011). https://doi.org/10.1016/j.biomaterials.2010.12.016
Kang, Y., Kim, S., Bishop, J., Khademhosseini, A., Yang, Y.: The osteogenic differentiation of human bone marrow MSCs on HUVEC-derived ECM and β-TCP scaffold. Biomaterials. 33, 6998–7007 (2012). https://doi.org/10.1016/j.biomaterials.2012.06.061
Zeitouni, S., Krause, U., Clough, B.H., Halderman, H., Falster, A., Blalock, D.T., Chaput, C.D., Sampson, H.W., Gregory, C.A.: Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci. Transl. Med. 4, 132–155 (2012). https://doi.org/10.1126/scitranslmed.3003396
Yang, Y., Lin, H., Shen, H., Wang, B., Lei, G., Tuan, R.S.: Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 69, 71–82 (2018). https://doi.org/10.1016/j.actbio.2017.12.043
Zhang, W., Zhu, Y., Li, J., Guo, Q., Peng, J., Liu, S., Yang, J., Wang, Y.: Cell-derived extracellular matrix: basic characteristics and current applications in orthopedic tissue engineering. Tissue Eng. B Rev. 22, 193–207 (2016). https://doi.org/10.1089/ten.teb.2015.0290
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F.C., Krause, D.S., Deans, R.J., Keating, A., Prockop, D.J., Horwitz, E.M.: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8, 315–317 (2006). https://doi.org/10.1080/14653240600855905
Murphy, M.B., Moncivais, K., Caplan, A.I.: Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine, (2013)
Jin, C.Z., Choi, B.H., Park, S.R., Min, B.H.: Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J. Biomed. Mater. Res. A. 92, 1567–1577 (2010). https://doi.org/10.1002/jbm.a.32419
Park, Y.B., Seo, S., Kim, J.A., Heo, J.C., Lim, Y.C., Ha, C.W.: Effect of chondrocyte-derived early extracellular matrix on chondrogenesis of placenta-derived mesenchymal stem cells. Biomed. Mater. 10, (2015). https://doi.org/10.1088/1748-6041/10/3/035014
Linhardt, R.J., Toida, T.: Role of glycosaminoglycans in cellular communication. Acc. Chem. Res. 37, 431–438 (2004). https://doi.org/10.1021/ar030138x
Gasimli, L., Linhardt, R.J., Dordick, J.S.: Proteoglycans in stem cells. Biotechnol. Appl. Biochem. 59, 65–76 (2012). https://doi.org/10.1002/bab.1002
Weyers, A., Linhardt, R.J.: Neoproteoglycans in tissue engineering. FEBS J. 280, 2511–2522 (2013). https://doi.org/10.1111/febs.12187
Wang, M., Liu, X., Lyu, Z., Gu, H., Li, D., Chen, H.: Glycosaminoglycans (GAGs) and GAG mimetics regulate the behavior of stem cell differentiation. Colloids Surf. B: Biointerfaces. 150, 175–182 (2017). https://doi.org/10.1016/j.colsurfb.2016.11.022
Papy-Garcia, D., Albanese, P.: Heparan sulfate proteoglycans as key regulators of the mesenchymal niche of hematopoietic stem cells. Glycoconj. J. 34, 377–391 (2017). https://doi.org/10.1007/s10719-017-9773-8
Gasimli, L., Hickey, A.M., Yang, B., Li, G., Dela Rosa, M., Nairn, A.V., Kulik, M.J., Dordick, J.S., Moremen, K.W., Dalton, S., Linhardt, R.J.: Changes in glycosaminoglycan structure on differentiation of human embryonic stem cells towards mesoderm and endoderm lineages. Biochim. Biophys. Acta, Gen. Subj. 1840, 1993–2003 (2014). https://doi.org/10.1016/j.bbagen.2014.01.007
Kjellén, L., Lindahl, U.: Specificity of glycosaminoglycan–protein interactions. Curr. Opin. Struct. Biol. 50, 101–108 (2018). https://doi.org/10.1016/j.sbi.2017.12.011
Ibrahimi, O.A., Zhang, F., Hrstka, S.C.L., Mohammadi, M., Linhardt, R.J.: Kinetic model for FGF, FGFR, and proteoglycan signal transduction complex assembly. Biochemistry. 43, 4724–4730 (2004). https://doi.org/10.1021/bi0352320
Cool, S.M., Nurcombe, V.: The osteoblast-heparan sulfate axis: control of the bone cell lineage. Int. J. Biochem. Cell Biol. 37, 1739–1745 (2005). https://doi.org/10.1016/j.biocel.2005.03.006
Uygun, B.E., Stojsih, S.E., Matthew, H.W.T.: Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng. A. 15, 3499–3512 (2009). https://doi.org/10.1089/ten.TEA.2008.0405
Dombrowski, C., Song, S.J., Chuan, P., Lim, X., Susanto, E., Sawyer, A.A., Woodruff, M.A., Hutmacher, D.W., Nurcombe, V., Cool, S.M.: Heparan sulfate mediates the proliferation and differentiation of rat mesenchymal stem cells. Stem Cells Dev. 18, 661–670 (2009). https://doi.org/10.1089/scd.2008.0157
Manton, K.J., Leong, D.F.M., Cool, S.M., Nurcombe, V.: Disruption of heparan and chondroitin sulfate signaling enhances mesenchymal stem cell-derived osteogenic differentiation via bone morphogenetic protein signaling pathways. Stem Cells. 25, 2845–2854 (2007). https://doi.org/10.1634/stemcells.2007-0065
Celikkin, N., Rinoldi, C., Costantini, M., Trombetta, M., Rainer, A., Święszkowski, W.: Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater. Sci. Eng. C. 78, 1277–1299 (2017). https://doi.org/10.1016/j.msec.2017.04.016
Pfeifer, C.G., Berner, A., Koch, M., Krutsch, W., Kujat, R., Angele, P., Nerlich, M., Zellner, J.: Higher ratios of hyaluronic acid enhance chondrogenic differentiation of human MSCs in a hyaluronic acid-gelatin composite scaffold. Materials (Basel). 9, (2016). https://doi.org/10.3390/ma9050381
Christiansen-Weber, T., Noskov, A., Cardiff, D., Garitaonandia, I., Dillberger, A., Semechkin, A., Gonzalez, R., Kern, R.: Supplementation of specific carbohydrates results in enhanced deposition of chondrogenic-specific matrix during mesenchymal stem cell differentiation. J. Tissue Eng. Regen. Med. 12, 1261–1272 (2018). https://doi.org/10.1002/term.2658
Amann, E., Wolff, P., Breel, E., van Griensven, M., Balmayor, E.R.: Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater. 52, 130–144 (2017). https://doi.org/10.1016/j.actbio.2017.01.064
Weyers, A., Yang, B., Yoon, D.S., Park, J.-H., Zhang, F., Lee, K.B., Linhardt, R.J.: A structural analysis of Glycosaminoglycans from lethal and nonlethal breast Cancer tissues: toward a novel class of Theragnostics for personalized medicine in oncology? OMICS 16, 79–89 (2012). https://doi.org/10.1089/omi.2011.0102
Heiskanen, A., Hirvonen, T., Salo, H., Impola, U., Olonen, A., Laitinen, A., Tiitinen, S., Natunen, S., Aitio, O., Miller-Podraza, H., Wuhrer, M., Deelder, A.M., Natunen, J., Laine, J., Lehenkari, P., Saarinen, J., Satomaa, T., Valmu, L.: Glycomics of bone marrow-derived mesenchymal stem cells can be used to evaluate their cellular differentiation stage. Glycoconj. J. 26, 367–384 (2009). https://doi.org/10.1007/s10719-008-9217-6
Hasehira, K., Hirabayashi, J., Tateno, H.: Structural and quantitative evidence of α2–6-sialylated N-glycans as markers of the differentiation potential of human mesenchymal stem cells. Glycoconj. J. 34, 797–806 (2017). https://doi.org/10.1007/s10719-016-9699-6
Kubaski, F., Osago, H., Mason, R.W., Yamaguchi, S., Kobayashi, H., Tsuchiya, M., Orii, T., Tomatsu, S.: Glycosaminoglycans detection methods : applications of mass spectrometry. Mol. Genet. Metab. 120, 67–77 (2017). https://doi.org/10.1016/j.ymgme.2016.09.005
Sun, X., Li, L., Overdier, K.H., Ammons, L.A., Douglas, I.S., Burlew, C.C., Zhang, F., Schmidt, E.P., Chi, L., Linhardt, R.J.: Analysis of Total human urinary glycosaminoglycan disaccharides by liquid chromatography-tandem mass spectrometry. Anal. Chem. 87, 6220–6227 (2015). https://doi.org/10.1021/acs.analchem.5b00913
Oguma, T., Tomatsu, S., Montano, A.M., Okazaki, O.: Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal. Biochem. 368, 79–86 (2007). https://doi.org/10.1016/j.ab.2007.05.016
Wang, C., Lang, Y., Li, Q., Jin, X., Li, G., Yu, G.: Glycosaminoglycanomic profiling of human milk in different stages of lactation by liquid chromatography-tandem mass spectrometry. Food Chem. 258, 231–236 (2018). https://doi.org/10.1016/j.foodchem.2018.03.076
Li, G., Li, L., Tian, F., Zhang, L., Xue, C., Linhardt, R.J.: Glycosaminoglycanomics of cultured cells using a rapid and sensitive LC-MS/MS approach. ACS Chem. Biol. 10, 1303–1310 (2015). https://doi.org/10.1021/acschembio.5b00011
Liu, X., Krishnamoorthy, D., Lin, L., Xue, P., Zhang, F., Chi, L., Linhardt, R.J., Iatridis, J.C.: A method for characterising human intervertebral disc glycosaminoglycan disaccharides using liquid chromatography-mass spectrometry with multiple reaction monitoring. Eur. Cell. Mater. 35, 117–131 (2018). https://doi.org/10.22203/eCM.v035a09
Dos Santos, F., Andrade, P.Z., Boura, J.S., Abecasis, M.M., da Silva, C.L., Cabral, J.M.S.: Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol. 223, 27–35 (2010). https://doi.org/10.1002/jcp.21987
Santhagunam, A., Dos Santos, F., Madeira, C., Salgueiro, J.B., Cabral, J.M.S.: Isolation and ex vivo expansion of synovial mesenchymal stromal cells for cartilage repair. Cytotherapy. 16, 440–453 (2013). https://doi.org/10.1016/j.jcyt.2013.10.010
Guneta, V., Zhou, Z., Tan, N.S., Sugii, S., Wong, M.T.C., Choong, C.: Recellularization of decellularized adipose tissue-derived stem cells: role of the cell-secreted extracellular matrix in cellular differentiation. Biomater. Sci. 6, 168–178 (2018). https://doi.org/10.1039/c7bm00695k
Hoshiba, T., Lu, H., Kawazoe, N., Yamada, T., Chen, G.: Effects of extracellular matrix proteins in chondrocyte-derived matrices on chondrocyte functions. Biotechnol. Prog. 29, 1331–1336 (2013). https://doi.org/10.1002/btpr.1780
Kaukonen, R., Jacquemet, G., Hamidi, H., Ivaska, J.: Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat. Protoc. 12, 2376–2390 (2017). https://doi.org/10.1038/nprot.2017.107
Ragelle, H., Naba, A., Larson, B.L., Zhou, F., Prijić, M., Whittaker, C.A., Del Rosario, A., Langer, R., Hynes, R.O., Anderson, D.G.: Comprehensive proteomic characterization of stem cell-derived extracellular matrices. Biomaterials. 128, 147–159 (2017). https://doi.org/10.1016/j.biomaterials.2017.03.008
Knudson, C.B., Knudson, W.: Cartilage proteoglycans. Semin. Cell Dev. Biol. 12, 69–78 (2001). https://doi.org/10.1007/978-3-319-29568-8_1
Roughle, P.J.: The structure and function of cartilage Proteoglicans. Eur. Cell. Mater. 12, 92–101 (2006)
Mouw, J.K., Case, N.D., Guldberg, R.E., Plaas, A.H.K., Levenston, M.E.: Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthr. Cartil. 13, 828–836 (2005). https://doi.org/10.1016/j.joca.2005.04.020
Lauder, R.M., Huckerby, T.N., Brown, G.M., Bayliss, M.T., Nieduszynski, I.a.: Age-related changes in the sulphation of the chondroitin sulphate linkage region from human articular cartilage aggrecan. Biochem. J. 358, 523–528 (2001). https://doi.org/10.1042/0264-6021:3580523
Sharma, A., Rees, D., Roberts, S., Kuiper, N.J.: A case study: glycosaminoglycan profiles of autologous chondrocyte implantation (ACI) tissue improve as the tissue matures. Knee. 24, 149–157 (2017). https://doi.org/10.1016/j.knee.2016.10.002
Acknowledgments
This work was supported by funding received by iBB-Institute for Bioengineering and Biosciences through Programa Operacional Regional de Lisboa 2020 (Project N. 007317), through the EU COMPETE Program and from National Funds through FCT-Portuguese Foundation for Science and Technology under the Programme grant UID/BIO/04565/2013 and by the European Union Framework Programme for Research and Innovation HORIZON 2020, under the Teaming Grant agreement No 739572 – The Discoveries Centre for Regenerative and Precision Medicine. This study was also supported by Center for Biotechnology and Interdisciplinary Studies-Rensselaer Polytechnic Institute funds and by the National Institutes of Health (Grant # DK111958). João C. Silva and Marta S. Carvalho would also like to acknowledge FCT for financial support through the scholarships SFRH/BD/105771/2014 and SFRH/BD/52478/2014, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This work does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 460 kb)
Rights and permissions
About this article
Cite this article
Silva, J.C., Carvalho, M.S., Han, X. et al. Compositional and structural analysis of glycosaminoglycans in cell-derived extracellular matrices. Glycoconj J 36, 141–154 (2019). https://doi.org/10.1007/s10719-019-09858-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-019-09858-2