Summary
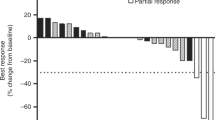
Background The MAPK pathway plays a central role in regulation of several cellular processes, and its dysregulation is a hallmark of biliary tract cancer (BTC). Binimetinib (MEK162), a potent, selective oral MEK1/2 inhibitor, was assessed in patients with advanced BTC. Patients and Methods An expansion cohort study in patients who received ≤1 line of therapy for advanced BTC was conducted after determination of the maximum tolerated dose in this Phase 1 trial. Patients received binimetinib 60 mg twice daily. The primary objectives were to characterize the safety profile and pharmacokinetics of binimetinib in advanced BTC. Secondary objectives included assessment of clinical efficacy, changes in weight and lean body mass, and pharmacodynamic effects. Tumor samples were assessed for mutations in relevant genes. Results Twenty-eight patients received binimetinib. Common adverse events (AEs) were mild, with rash (82%) and nausea (54%) being most common. Two patients experienced grade 4 AEs, one generalized edema and the other pulmonary embolism. The pharmacokinetics in this patient population were consistent with those previously reported (Bendell JC et al., Br J Cancer 2017;116:575-583). Twelve patients (43%) experienced stable disease and two had objective responses (1 complete response, 1 partial response) per Response Evaluation Criteria in Solid Tumors and stable metabolic disease by positron emission tomography/computed tomography. Most patients (18/25; 72%) did not have KRAS, BRAF, NRAS, PI3KCA, or PTEN mutations, nor was there correlation between mutation status and response. The average non-fluid weight gain was 1.3% for lean muscle and 4.7% for adipose tissue. Conclusion Binimetinib was well tolerated and showed promising evidence of activity in patients with BTC. Correlative studies suggested the potential for binimetinib to promote muscle gain in patients with BTC.

Similar content being viewed by others
References
Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD (2005) Cholangiocarcinoma. Lancet 366:1303–1314
Sadeghi S, Finn RS (2014) Systemic therapy for cholangiocarcinoma. Clin Liver Dis 3:86–89
Goydos JS, Brumfield AM, Frezza E et al (1998) Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg 227:398–404
Park J, Tadlock L, Gores GJ, Patel T (1999) Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology 30:1128–1133
Davies H, Bignell GR, Cox C et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954
Nakazawa K, Dobashi Y, Suzuki S et al (2005) Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol 206:356–365
Bennasroune A, Gardin A, Aunis D et al (2004) Tyrosine kinase receptors as attractive targets of cancer therapy. Crit Rev Oncol Hematol 50:23–38
McCubrey JA, May WS, Duronio V, Mufson A (2000) Serine/threonine phosphorylation in cytokine signal transduction. Leukemia 14:9–21
Bekaii-Saab T, Phelps MA, Li X et al (2011) Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 29:2357–2363
Prado CM, Bekaii-Saab T, Doyle LA et al (2012) Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 106:1583–1586
Bendell JC, Javle M, Bekaii-Saab TS et al (2017) A phase 1 dose-escalation and expansion study of binimetinib (MEK162), a potent and selective oral MEK1/2 inhibitor. Br J Cancer 116:575–583
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Shen W, Punyanitya M, Wang Z et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 97:2333–2338
Prado CM, Birdsell LA, Baracos VE (2009) The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 3:269–275
Prado CM, Sawyer MB, Ghosh S et al (2013) Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 98:1012–1019
Dry JR, Pavey S, Pratilas CA et al (2010) Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244). Cancer Res 70:2264–2273
Gores GJ (2003) Cholangiocarcinoma: current concepts and insights. Hepatology 37:961–969
Mon NN, Kokuryo T, Hamaguchi M (2009) Inflammation and tumor progression: a lesson from TNF-alpha-dependent FAK signaling in cholangiocarcinoma. Methods Mol Biol 512:279–293
Okada K, Shimizu Y, Nambu S et al (1994) Interleukin-6 functions as an autocrine growth factor in a cholangiocarcinoma cell line. J Gastroenterol Hepatol 9:462–467
Maemura K, Natsugoe S, Takao S (2014) Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci 21:754–760
Tai YT, Fulciniti M, Hideshima T et al (2007) Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis. Blood 110:1656–1663
Zhang D, Zheng H, Zhou Y et al (2007) Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer 7:45
Zhang D, Zhou Y, Wu L et al (2008) Association of IL-6 gene polymorphisms with cachexia susceptibility and survival time of patients with pancreatic cancer. Ann Clin Lab Sci 38:113–119
Funding
This work was supported by Array BioPharma Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. R. S. Finn is a consultant for Bayer, Novartis, Pfizer Inc., Bristol-Myers Squibb. Dr. D. H. Ahn is a consultant for Merrimack. Dr. A. Patnaik receives institutional research funding from Array BioPharma Inc. R. Chavira, J. Christy-Bittel, and Dr. Barrett are current or former employees, and may own stock in, Array BioPharma Inc. All remaining authors have declared no conflicts of interest.
Additional information
Key message: MAPK pathway dysregulation is common in biliary tract cancer and is a key mechanism for tumor proliferation and treatment resistance. Its inhibition represents a novel, relevant treatment strategy in this disease. Binimetinib was well tolerated and showed promising clinical activity. Future studies assessing biomarkers will help determine patients that are likely to benefit from binimetinib.
Electronic supplementary material
ESM 1
(PDF 101 kb)
Rights and permissions
About this article
Cite this article
Finn, R.S., Ahn, D.H., Javle, M.M. et al. Phase 1b investigation of the MEK inhibitor binimetinib in patients with advanced or metastatic biliary tract cancer. Invest New Drugs 36, 1037–1043 (2018). https://doi.org/10.1007/s10637-018-0600-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0600-2