Abstract
Purpose
We aimed to investigate the genomic profile of breast sarcomas (BS) and compare with that of malignant phyllodes tumours (MPT).
Methods
DNA was extracted from formalin-fixed, paraffin-embedded (FFPE) specimens from 17 cases of BS diagnosed at Singapore General Hospital from January 1991 to December 2014. Targeted deep sequencing and copy number variation (CNV) analysis on 16 genes, which included recurrently mutated genes in phyllodes tumours and genes associated with breast cancer, were performed on these samples. Genetic alterations (GA) observed were summarised and analysed.
Results
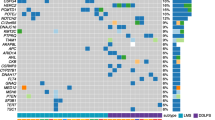
Nine cases met the quality control requirements for both targeted deep sequencing and CNV analysis. Three (33.33%) were angiosarcomas and 6 (66.67%) were non-angiosarcomas. In the non-angiosarcoma group, 83.33% (n = 5) of the patients had GA in the TERT gene. The other commonly mutated genes in this group of tumours were MED12 (n = 4, 66.67%), BCOR (n = 4, 66.67%), KMT2D (n = 3, 50%), FLNA (n = 3, 50%) and NF1 (n = 3, 50%). In contrast, none of the angiosarcomas had mutations or copy number alterations in TERT, MED12, BCOR, FLNA or NF1. Eighty percent of patients with GA in TERT (n = 5) had concurrent mutations in MED12. Sixty percent (n = 3) of these cases also demonstrated GA in NF1, PIK3CA or EGFR which are known cancer driver genes.
Conclusions
The non-angiosarcoma group of BS was found to share similar GA as those described for MPT, which may suggest a common origin and support their consideration as a similar group of tumours with regard to management and prognostication.


Similar content being viewed by others
References
Pollard SG, Marks PV, Temple LN, Thompson HH (1990) Breast sarcoma. A clinicopathologic review of 25 cases. Cancer 66:941–944. https://doi.org/10.1002/1097-0142(19900901)66:5%3C941::AID-CNCR2820660522%3E3.0.CO;2-B
Terrier P, Terrier-Lacombe MJ, Mouriesse H, Friedman S, Spielmann M, Contesso G (1989) Primary breast sarcoma: a review of 33 cases with immunohistochemistry and prognostic factors. Breast Cancer Res Treat 13(1):39–48. https://doi.org/10.1007/BF01806549
Lim SZ, Selvarajan S, Thike AA, Nasir ND, Tan BK, Ong KW, Tan PH (2016) Breast sarcomas and malignant phyllodes tumours: comparison of clinicopathological features, treatment strategies, prognostic factors and outcomes. Breast Cancer Res Treat 159:229–244. https://doi.org/10.1007/s10549-016-3946-1
Bousquet G, Confavreux C, Magné N, de Lara CT, Poortmans P, Senkus E, de Lafontan B, Bolla M, Largillier R, Lagneau E, Kadish S, Lemanski C, Ozsahin M, Belkacémi Y (2007) Outcome and prognostic factors in breast sarcoma: a multicenter study from the rare cancer network. Radiother Oncol 85(3):355–361. https://doi.org/10.1016/j.radonc.2007.10.015
Johnstone PA, Pierce LJ, Merino MJ, Yang JC, Epstein AH, DeLaney TF (1993) Primary soft tissue sarcomas of the breast: local-regional control with post-operative radiotherapy. Int J Radiat Oncol Biol Phys 27(3):671–675. https://doi.org/10.1016/0360-3016(93)90395-C
Toesca A, Spitaleri G, De Pas T, Botteri E, Gentilini O, Bottiglieri L, Rotmentsz N, Sangalli C, Marrazzo E, Cassano E, Veronesi P, Rietjens M, Luini A (2012) Sarcoma of the breast: outcome and reconstructive options. Clin Breast Cancer 12(6):438–444. https://doi.org/10.1016/j.clbc.2012.09.008
Adem C, Reynolds C, Ingle JN, Nascimento AG (2004) Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer 91(2):237–241. https://doi.org/10.1038/sj.bjc.6601920
Barnes L, Pietruszka M (1977) Sarcomas of the breast: a clinicopathologic analysis of ten cases. Cancer 40(4):1577–1585. https://doi.org/10.1002/1097-0142(197710)40:4%3C1577::AID-CNCR2820400430%3E3.0.CO;2-D
Barrow BJ, Janjan NA, Gutman H, Benjamin RS, Allen P, Romsdahl MM, Ross MI, Pollock RE (1999) Role of radiotherapy in sarcoma of the breast–a retrospective review of the M.D. Anderson experience. Radiother Oncol 52(2):173–178. https://doi.org/10.1016/S0167-8140(99)00070-5
Fields RC, Aft RL, Gillanders WE, Eberlein TJ, Margenthaler JA (2008) Treatment and outcomes of patients with primary breast sarcoma. Am J Surg 196(4):559–561. https://doi.org/10.1016/j.amjsurg.2008.06.010
North JH Jr, McPhee M, Arredondo M, Edge SB (1998) Sarcoma of the breast: implications of the extent of local therapy. Am Surg 64(11):1059–1061
Pandey M, Mathew A, Abraham EK, Rajan B (2004) Primary sarcoma of the breast. J Surg Oncol 87(3):121–125. https://doi.org/10.1002/jso.20110
Stanley MW, Tani EM, Horwitz CA, Tulman S, Skoog L (1988) Primary spindle-cell sarcomas of the breast: diagnosis by fine-needle aspiration. Diagn Cytopathol 4(3):244–249. https://doi.org/10.1002/dc.2840040313
Surov A, Holzhausen HJ, Ruschke K, Spielmann RP (2011) Primary breast sarcoma: prevalence, clinical signs, and radiological features. Acta Radiol 52(6):597–601. https://doi.org/10.1258/ar.2011.100468
Lim SZ, Ong KW, Tan BK, Selvarajan S, Tan PH (2016) Sarcoma of the breast: an update on a rare entity. J Clin Pathol 69(5):373–381. https://doi.org/10.1136/jclinpath-2015-203545
Gao P, Seebacher NA, Hornicek F, Guo Z, Duan Z (2018) Advances in sarcoma gene mutations and therapeutic targets. Cancer Treat Rev 62:98–109. https://doi.org/10.1016/j.ctrv.2017.11.001
Tan PH, Tse GM, Lee A, Simpson J, Hanby A (2012) Fibroepithelial tumours. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ (eds) WHO classification of tumours of the breast. IARC Press, Lyon, pp 142–147
Sawyer EJ, Poulsom R, Hunt FT, Jeffery R, Elia G, Ellis IO, Ellis P, Tomlinson IP, Hanby AM (2003) Malignant phyllodes tumours show stromal overexpression of c-myc and c-kit. J Pathol 200(1):59–64. https://doi.org/10.1002/path.1318
Sawyer EJ, Hanby AM, Rowan AJ, Gillett CE, Thomas RE, Poulsom R, Lakhani SR, Ellis IO, Ellis P, Tomlinson IP (2002) The Wnt pathway, epithelial-stromal interactions, and malignant progression in phyllodes tumours. J Pathol 196(4):437–444. https://doi.org/10.1002/path.1067
Sawyer EJ, Hanby AM, Ellis P, Lakhani SR, Ellis IO, Boyle S, Tomlinson IP (2000) Molecular analysis of phyllodes tumors reveals distinct changes in the epithelial and stromal components. Am J Pathol 156(3):1093–1098. https://doi.org/10.1016/S0002-9440(10)64977-2
Karim RZ, Gerega SK, Yang YH, Horvath L, Spillane A, Carmalt H, Scolyer RA, Lee CS (2009) Proteins from the Wnt pathway are involved in the pathogenesis and progression of mammary phyllodes tumours. J Clin Pathol 62(11):1016–1020. https://doi.org/10.1136/jcp.2009.066977
Karim RZ, Scolyer RA, Tse GM, Tan PH, Putti TC, Lee CS (2009) Pathogenic mechanisms in the initiation and progression of mammary phyllodes tumours. Pathology 41(2):105–117. https://doi.org/10.1080/00313020802579342
Yoshida M, Ogawa R, Yoshida H, Maeshima A, Kanai Y, Kinoshita T, Hiraoka N, Sekine S (2015) TERT promoter mutations are frequent and show association with MED12 mutations in phyllodes tumors of the breast. Br J Cancer 113(8):1244–1248. https://doi.org/10.1038/bjc.2015.326
Tan J, Ong CK, Lim WK, Ng CC, Thike AA, Ng LM, Rajasegaran V, Myint SS, Nagarajan S, Thangaraju S, Dey S, Nasir ND, Wijaya GC, Lim JQ, Huang D, Li Z, Wong BH, Chan JY, McPherson JR, Cutcutache I, Poore G, Tay ST, Tan WJ, Putti TC, Ahmad BS, Iau P, Chan CW, Tang AP, Yong WS, Madhukumar P, Ho GH, Tan VK, Wong CY, Hartman M, Ong KW, Tan BK, Rozen SG, Tan P, Tan PH, Teh BT (2015) Genomic landscapes of breast fibroepithelial tumors. Nat Genet 47(11):1341–1345. https://doi.org/10.1038/ng.3409
Nozad S, Sheehan CE, Gay LM, Elvin JA, Vergilio JA, Suh J, Ramkissoon S, Schrock AB, Hirshfield KM, Ali N, Ganesan S, Ali SM, Miller VA, Stephens PJ, Ross JS, Chung JH (2017) Comprehensive genomic profiling of malignant phyllodes tumors of the breast. Breast Cancer Res Treat 162(3):597–602. https://doi.org/10.1007/s10549-017-4156-1
McGowan TS, Cummings BJ, O’Sullivan B, Catton CN, Miller N, Panzarella T (2000) An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys 46(2):383–390. https://doi.org/10.1016/S0360-3016(99)00444-7
McGregor GI, Knowling MA, Este FA (1994) Sarcoma and Cystosarcoma phyllodes tumors of the breast–a retrospective review of 58 cases. Am J Surg 167(5):477–480. https://doi.org/10.1016/0002-9610(94)90238-0
Confavreux C, Lurkin A, Mitton N, Blondet R, Saba C, Ranchère D, Sunyach MP, Thiesse P, Biron P, Blay JY, Ray-Coquard I (2006) Sarcomas and malignant phyllodes tumours of the breast–a retrospective study. Eur J Cancer 42(16):2715–2721. https://doi.org/10.1016/j.ejca.2006.05.040
Wang F, Jia Y, Tong Z (2015) Comparison of the clinical and prognostic features of primary breast sarcomas and malignant phyllodes tumor. Jpn J Clin Oncol 45(2):146–152. https://doi.org/10.1093/jjco/hyu177
1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR (2015) A global reference for human genetic variation. Nature 526(7571):68–74. https://doi.org/10.1038/nature15393
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26(5):589–595. https://doi.org/10.1093/bioinformatics/btp698
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25(16):2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Garrison E, Marth G (2012) Haplotype-based variant detection from short-read sequencing. https://arxiv.org/abs/1207.3907. Accessed 5 Sept 2018
Yang H, Wang K (2015) Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat Protoc 10(10):1556–1566. https://doi.org/10.1038/nprot.2015.105
Thorvaldsdóttir H, Robinson JT, Mesirov JP (2013) Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14(2):178–192. https://doi.org/10.1093/bib/bbs017
Jung H-S, Lefferts JA, Gregory J, Tsongalis (2017) Utilization of the oncoscan microarray assay in cancer diagnostics. Appl Cancer Res 37(1):1. https://doi.org/10.1186/s41241-016-0007-3
Schmidt J, Liu B, Ghent M, Bolstad B, Siddiqui F, Abdueva D, Marjanovic M, Saplosky R, Shukla A, Venkatapathy S, Chen C, Bruckner C, Huynh V, Liu L, Suyenaga K, Weaver P, Greenfield L, Fung E (2014) A new method for high fidelity copy number analysis in solid tumor samples and its implementation in the OncoScan ™ FFPE assay kit. American Society of Human Genetics. http://www.ashg.org/2014meeting/abstracts/fulltext/f140122485.htm. Accessed 6 May 2018
Zelek L, Llombart-Cussac A, Terrier P, Pivot X, Guinebretiere JM, Le Pechoux C, Tursz T, Rochard F, Spielmann M, Le Cesne A (2003) Prognostic factors in primary breast sarcomas: a series of patients with long-term follow-up. J Clin Oncol 21(13):2583–2588. https://doi.org/10.1200/JCO.2003.06.080
Fraga-Guedes C, André S, Mastropasqua MG, Botteri E, Toesca A, Rocha RM, Peradze N, Rotmensz N, Viale G, Veronesi P, Gobbi H (2015) Angiosarcoma and atypical vascular lesions of the breast: diagnostic and prognostic role of MYC gene amplification and protein expression. Breast Cancer Res Treat 151(1):131–140. https://doi.org/10.1007/s10549-015-3379-2
Manner J, Radlwimmer B, Hohenberger P, Mössinger K, Küffer S, Sauer C, Belharazem D, Zettl A, Coindre JM, Hallermann C, Hartmann JT, Katenkamp D, Katenkamp K, Schöffski P, Sciot R, Wozniak A, Lichter P, Marx A, Ströbel P (2010) MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 176(1):34–39. https://doi.org/10.2353/ajpath.2010.090637
Italiano A, Thomas R, Breen M, Zhang L, Crago AM, Singer S, Khanin R, Maki RG, Mihailovic A, Hafner M, Tuschl T, Antonescu CR (2012) The miR-17-92 cluster and its target THBS1 are differentially expressed in angiosarcomas dependent on MYC amplification. Genes Chromosomes Cancer 51(6):569–578. https://doi.org/10.1002/gcc.21943
Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR (2011) Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer 50(1):25–33. https://doi.org/10.1002/gcc.20827
Thibodeau BJ, Lavergne V, Dekhne N, Benitez P, Amin M, Ahmed S, Nakamura JL, Davidson PR, Nakamura AO, Grills IS, Chen PY, Wobb J, Wilson GD (2018) Mutational landscape of radiation-associated angiosarcoma of the breast. Oncotarget 9(11):10042–10053. https://doi.org/10.18632/oncotarget.24273
Acknowledgements
This work was supported by funding from the Singapore General Hospital Research Grant 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research board.
Rights and permissions
About this article
Cite this article
Lim, S.Z., Ng, C.C.Y., Rajasegaran, V. et al. Genomic profile of breast sarcomas: a comparison with malignant phyllodes tumours. Breast Cancer Res Treat 174, 365–373 (2019). https://doi.org/10.1007/s10549-018-5067-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-5067-5