Abstract
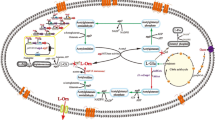
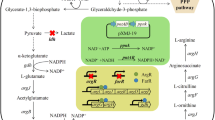
N-acetyl-l-glutamate kinase (NAGK) catalyzes the second step of l-arginine biosynthesis and is inhibited by l-arginine in Corynebacterium crenatum. To ascertain the basis for the arginine sensitivity of CcNAGK, residue E19 which located at the entrance of the Arginine-ring was subjected to site-saturated mutagenesis and we successfully illustrated the inhibition-resistant mechanism. Typically, the E19Y mutant displayed the greatest deregulation of l-arginine feedback inhibition. An equally important strategy is to improve the catalytic activity and thermostability of CcNAGK. For further strain improvement, we used site-directed mutagenesis to identify mutations that improve CcNAGK. Results identified variants I74V, F91H and K234T display higher specific activity and thermostability. The l-arginine yield and productivity of the recombinant strain C. crenatum SYPA-EH3 (which possesses a combination of all four mutant sites, E19Y/I74V/F91H/K234T) reached 61.2 and 0.638 g/L/h, respectively, after 96 h in 5 L bioreactor fermentation, an increase of approximately 41.8% compared with the initial strain.







Similar content being viewed by others
References
Alvares TS, Meirelles CM, Bhambhani YN, Paschoalin VM, Gomes PS (2011) l-Arginine as a potential ergogenic aid in healthy subjects. Sports Med 41:233–248. doi:10.2165/11538590-000000000-00000
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chaudhary S, Vats ID, Chopra M, Biswas P, Pasha S (2009) Effect of varying chain length between P(1) and P(1′) position of tripeptidomimics on activity of angiotensin-converting enzyme inhibitors. Bioorg Med Chem Lett 19:4364–4366. doi:10.1016/j.bmcl.2009.05.079
Cunin R, Glansdorff N, Pierard A, Stalon V (1986) Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev 50:314–352
Duperray F, Jezequel D, Ghazi A, Letellier L, Shechter E (1992) Excretion of glutamate from Corynebacterium glutamicum triggered by amine surfactants. Biochim Biophys Acta 1103:250–258
Haas D, Kurer V, Leisinger T (1972) N-acetylglutamate synthetase of Pseudomonas aeruginosa. An assay in vitro and feedback inhibition by arginine. Eur J Biochem 31:290–295
Holatko J, Elisakova V, Prouza M, Sobotka M, Nesvera J, Patek M (2009) Metabolic engineering of the l-valine biosynthesis pathway in Corynebacterium glutamicum using promoter activity modulation. J Biotechnol 139:203–210. doi:10.1016/j.jbiotec.2008.12.005
Huang Y, Li C, Zhang H, Liang S, Han S, Lin Y, Yang X, Zheng S (2016) Monomeric Corynebacterium glutamicum N-acetyl glutamate kinase maintains sensitivity to l-arginine but has a lower intrinsic catalytic activity. Appl Microbiol Biotechnol 100:1789–1798. doi:10.1007/s00253-015-7065-4
Huang Y, Zhang H, Tian H, Li C, Han S, Lin Y, Zheng S (2015) Mutational analysis to identify the residues essential for the inhibition of N-acetyl glutamate kinase of Corynebacterium glutamicum. Appl Microbiol Biotechnol 99:7527–7537. doi:10.1007/s00253-015-6469-5
Ikeda M, Mitsuhashi S, Tanaka K, Hayashi M (2009) Reengineering of a Corynebacterium glutamicum l-arginine and l-citrulline producer. Appl Environ Microbiol 75:1635–1641. doi:10.1128/AEM.02027-08
Kisumi M, Kato J, Sugiura M, Chibata I (1971) Production of l-arginine by arginine hydroxamate-resistant mutants of Bacillus subtilis. Appl Microbiol 22:987–991
MacArthur MW, Thornton JM (1991) Influence of proline residues on protein conformation. J Mol Biol 218:397–412
Man Z, Xu M, Rao Z, Guo J, Yang T, Zhang X, Xu Z (2016) Systems pathway engineering of Corynebacterium crenatum for improved l-arginine production. Sci Rep 6:28629. doi:10.1038/srep28629
Marco-Marı́n C, Ramón-Maiques S, Tavárez S, Rubio V (2003) Site-directed mutagenesis of Escherichia coli acetylglutamate kinase and aspartokinase III probes the catalytic and substrate-binding mechanisms of these amino acid kinase family enzymes and allows three-dimensional modelling of aspartokinase. J Mol Biol 334:459–476. doi:10.1016/j.jmb.2003.09.038
Park JH, Lee SY (2008) Towards systems metabolic engineering of microorganisms for amino acid production. Curr Opin Biotechnol 19:454–460. doi:10.1016/j.copbio.2008.08.007
Park SH, Kim HU, Kim TY, Park JS, Kim SS, Lee SY (2014) Metabolic engineering of Corynebacterium glutamicum for l-arginine production. Nat Commun 5:4618. doi:10.1038/ncomms5618
Ramon-Maiques S, Fernandez-Murga ML, Gil-Ortiz F, Vagin A, Fita I, Rubio V (2006) Structural bases of feed-back control of arginine biosynthesis, revealed by the structures of two hexameric N-acetylglutamate kinases, from Thermotoga maritima and Pseudomonas aeruginosa. J Mol Biol 356:695–713. doi:10.1016/j.jmb.2005.11.079
Schendzielorz G, Dippong M, Grunberger A, Kohlheyer D, Yoshida A, Binder S, Nishiyama C, Nishiyama M, Bott M, Eggeling L (2014) Taking control over control: use of product sensing in single cells to remove flux control at key enzymes in biosynthesis pathways. ACS Synth Biol 3:21–29. doi:10.1021/sb400059y
Schmidt AE, Sun MF, Ogawa T, Bajaj SP, Gailani D (2008) Functional role of residue 193 (chymotrypsin numbering) in serine proteases: influence of side chain length and beta-branching on the catalytic activity of blood coagulation factor XIa. Biochemistry 47:1326–1335. doi:10.1021/bi701594j
Schneider J, Niermann K, Wendisch VF (2011) Production of the amino acids l-glutamate, l-lysine, l-ornithine and l-arginine from arabinose by recombinant Corynebacterium glutamicum. J Biotechnol 154:191–198. doi:10.1016/j.jbiotec.2010.07.009
Shin JH, Lee SY (2014) Metabolic engineering of microorganisms for the production of l-arginine and its derivatives. Microb Cell Fact 13:166. doi:10.1186/s12934-014-0166-4
Tauch A, Kirchner O, Loffler B, Gotker S, Puhler A, Kalinowski J (2002) Efficient electrotransformation of corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Curr Microbiol 45:362–367. doi:10.1007/s00284-002-3728-3
Xu H, Dou W, Xu H, Zhang X, Rao Z, Shi Z, Xu Z (2009) A two-stage oxygen supply strategy for enhanced l-arginine production by Corynebacterium crenatum based on metabolic fluxes analysis. Biochem Eng J 43:41–51. doi:10.1016/j.bej.2008.08.007
Xu M, Rao Z, Dou W, Jin J, Xu Z (2012) Site-directed mutagenesis studies on the l-arginine-binding sites of feedback inhibition in N-acetyl-l-glutamate kinase (NAGK) from Corynebacterium glutamicum. Curr Microbiol 64:164–172. doi:10.1007/s00284-011-0042-y
Xu M, Rao Z, Dou W, Yang J, Jin J, Xu Z (2012) Site-directed mutagenesis and feedback-resistant N-acetyl-l-glutamate kinase (NAGK) increase Corynebacterium crenatum l-arginine production. Amino Acids 43:255–266. doi:10.1007/s00726-011-1069-x
Yu P, Xu M (2012) Enhancing the enzymatic activity of the endochitinase by the directed evolution and its enzymatic property evaluation. Process Biochem 47:1089–1094. doi:10.1016/j.procbio.2012.03.015
Zhang B, Wan F, Qiu YL, Chen XL, Tang L, Chen JC, Xiong YH (2015) Increased l-arginine production by site-directed mutagenesis of N-acetyl-l-glutamate kinase and proB gene deletion in Corynebacterium crenatum. Biomed Environ Sci 28:864–874. doi:10.3967/bes2015.120
Zhao Q, Luo Y, Dou W, Zhang X, Zhang X, Zhang W, Xu M, Geng Y, Rao Z, Xu Z (2016) Controlling the transcription levels of argGH redistributed l-arginine metabolic flux in N-acetylglutamate kinase and ArgR-deregulated Corynebacterium crenatum. J Ind Microbiol Biotechnol 43:55–66. doi:10.1007/s10295-015-1692-8
Acknowledgements
This work was supported by the High-tech Research and Development Programs of China (2015AA021004), the National Natural Science Foundation of China (31300028), the Jiangsu Provincial National Basic Research Program (BK20150002, BK20130137), the Research Project of Chinese Ministry of Education (113033A), the Program Funded by China Scholarship Council, the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project (No. 111-2-06), and the Jiangsu province “Collaborative Innovation Center for Advanced Industrial Fermentation” industry development program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Xu, M., Ge, X. et al. Reengineering of the feedback-inhibition enzyme N-acetyl-l-glutamate kinase to enhance l-arginine production in Corynebacterium crenatum . J Ind Microbiol Biotechnol 44, 271–283 (2017). https://doi.org/10.1007/s10295-016-1885-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1885-9