Abstract
Background
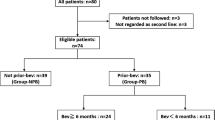
Dose modification of chemotherapy for metastatic colorectal cancer (MCRC) is often needed, especially in second-line and later-line treatments due to adverse events of previous treatment and poor patient condition. No study has focused on ramucirumab plus modified dose of FOLFIRI for MCRC, and whether low relative dose intensity (RDI) affects treatment efficacy has not been clarified.
Methods
MCRC patients who received ramucirumab plus FOLFIRI, which consisted of 150 mg/m2 of irinotecan, at six institutions were retrospectively analyzed.
Results
A total of 43 patients were assessed. Median age was 63 years, and 22 patients (51%) were women. Twenty-six patients (60%) were given ramucirumab plus FOLFIRI as second-line therapy, and 17 (40%) as third or later-line. The median relative dose intensity (RDI) of irinotecan was 60.6%, which is lower than that in the pivotal phase 3 study (RAISE), and other agents showed the same trend. Median progression-free survival was 4.8 [95% confidence interval (CI) 3.2–5.7] months for all patients, 5.4 (95% CI 3.5–7.2) months for second-line patients, and 2.8 (95% CI 1.6–5.8) months for third or later-line patients. Median overall survival was 17.3 (95% CI 11.5–22.4) months for all patients. Patients with irinotecan RDI less than 60% showed similar treatment efficacy. Hematological toxicities of grade 3 or worse were observed in 21 patients, but all were manageable.
Conclusion
Low RDI did not compromise the treatment efficacy of ramucirumab plus modified FOLFIRI for MCRC patients.


Similar content being viewed by others
References
WHO cancer today (2018) http://gco.iarc.fr/today/online-analysis-multi-bars?mode=cancer&mode_population=continents&population=900&sex=0&cancer=29&type=1&statistic=0&prevalence=0&color_palette=default. Accessed 29 Oct 2018
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Saltz LB, Clarke S, Díaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Heinemann V, von Weikersthal LF, Decker T et al (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1065–1075
Department of Health and Human Services Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research (2014) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125477Orig1s000PharmR.pdf. Accessed 29 Oct 2018
Tabernero J, Yoshino T, Cohn AL et al (2015) Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 16:499–508
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Cancer Therapy Evaluation Program, National Cancer Institute (2010) Common Terminology Criteria for Adverse Events (CTCAE) v4.0
Yoshino T, Obermannová R, Bodoky G et al (2017) Baseline carcinoembryonic antigen as a predictive factor of ramucirumab efficacy in RAISE, a second-line metastatic colorectal carcinoma phase III trial. Eur J Cancer 78:61–69
Grothey A, Yoshino T, Bodoky G et al (2018) Association of baseline absolute neutrophil counts and survival in patients with metastatic colorectal cancer treated with second-line antiangiogenic therapies: exploratory analyses of the RAISE trial and validation in an electronic medical record data set. ESMO Open 3(3):e000347
Nakayama G, Tanaka C, Uehara K et al (2014) The impact of dose/time modification in irinotecan- and oxaliplatin-based chemotherapies on outcomes in metastatic colorectal cancer. Cancer Chemother Pharmacol 73(4):847–855
Bennouna J, Sastre J, Arnold D et al (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14:29–37
Van Cutsem E, Tabernero J, Lakomy R et al (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30:3499–3506
Tabernero J, Hozak RR, Yoshino T et al (2018) Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann Oncol 29:602–609
Wirapati P, Pomella V, Vandenbosch B et al (2017) VELOUR trial biomarkers update: impact of RAS, BRAF, and sidedness on aflibercept activity. Ann Oncol 1(suppl_3):28
André T, Blons H, Mabro M et al (2013) Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Ann Oncol 24:412–419
Ivanova JI, Saverno KR, Sung J et al (2017) Real-world treatment patterns and effectiveness among patients with metastatic colorectal cancer treated with ziv-aflibercept in community oncology practices in the USA. Med Oncol 34:193
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307
Akiyama K, Ohga N, Hida Y et al (2012) Tumor endothelial cells acquire drug resistance by MDR1 up-regulation via VEGF signaling in tumor microenvironment. Am J Pathol 180:1283–1293
Ziegler BL, Valtieri M, Porada GA et al (1999) KDR receptor: a key marker defining hematopoietic stem cells. Science 285:1553–1558
Yoshino T, Yamazaki K, Gotoh M et al (2015) Safety and pharmacokinetics of second-line ramucirumab plus FOLFIRI in Japanese Patients with metastatic colorectal carcinoma. Anticancer Res 35:4003–4007
Komiyama S, Kato K, Inokuchi Y et al (2018) Bevacizumab combined with platinum-taxane chemotherapy as first-line treatment for advanced ovarian cancer: a prospective observational study of safety and efficacy in Japanese patients (JGOG3022 trial). Int J Clin Oncol. https://doi.org/10.1007/s10147-018-1319-y
Prager GW, Braemswig KH, Martel A et al (2014) Baseline carcinoembryonic antigen (CEA) serum levels predict bevacizumab-based treatment response in metastatic colorectal cancer. Cancer Sci 105:996–1001
Watt DG, Martin JC, Park JH et al (2015) Neutrophil count is the most important prognostic component of the differential white cell count in patients undergoing elective surgery for colorectal cancer. Am J Surg 210:24–30
Acknowledgements
The authors would like to thank all the participating patients and medical staff who treated the patients in each institution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Akitaka Makiyama have received a speaker honorarium from Eli Lilly. Taito Esaki has received a speaker honorarium from Daiichi Sankyo and Eli Lilly and has received research grants from Daiichi Sankyo. Koichi Akashi has received speaker honorarium from Kyowa Hakko Kirin. Koichi Akashi has received research grants from Kyowa Hakko Kirin, Yakult, Eli Lilly and Daiichi Sankyo. Eishi Baba has received a speaker honorarium and research grants from Eli Lilly. The other authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoshihiro, T., Kusaba, H., Makiyama, A. et al. Efficacy and safety of ramucirumab plus modified FOLFIRI for metastatic colorectal cancer. Int J Clin Oncol 24, 508–515 (2019). https://doi.org/10.1007/s10147-018-01391-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-01391-w