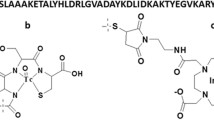
Abstract
Molecular imaging of human epidermal growth factor receptor type 2 (HER2) expression has drawn significant attention because of the unique role of the HER2 gene in diagnosis, therapy and prognosis of human breast cancer. In our previous research, a novel cyclic 2-helix small protein, MUT-DS, was discovered as an anti-HER2 Affibody analog with high affinity through rational protein design and engineering. MUT-DS was then evaluated for positron emission tomography (PET) of HER2-positive tumor by labeling with two radionuclides, 68Ga and 18F, with relatively short half-life (t 1/2 < 2 h). In order to fully study the in vivo behavior of 2-helix small protein and demonstrate that it could be a robust platform for labeling with a variety of radionuclides for different applications, in this study, MUT-DS was further radiolabeled with 64Cu or 111In and evaluated for in vivo targeting of HER2-positive tumor in mice. Design 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) conjugated MUT-DS (DOTA–MUT-DS) was chemically synthesized using solid phase peptide synthesizer and I2 oxidation. DOTA–MUT-DS was then radiolabeled with 64Cu or 111In to prepare the HER2 imaging probe (64Cu/111In-DOTA–MUT-DS). Both biodistribution and microPET imaging of the probe were evaluated in nude mice bearing subcutaneous HER2-positive SKOV3 tumors. DOTA–MUT-DS could be successfully synthesized and radiolabeled with 64Cu or 111In. Biodistribution study showed that tumor uptake value of 64Cu or 111In-labeled DOTA–MUT-DS was 4.66 ± 0.38 or 2.17 ± 0.15%ID/g, respectively, in nude mice bearing SKOV3 xenografts (n = 3) at 1 h post-injection (p.i.). Tumor-to-blood and tumor-to-muscle ratios for 64Cu-DOTA-MUT-DS were attained to be 3.05 and 3.48 at 1 h p.i., respectively, while for 111In-DOTA–MUT-DS, they were 2.04 and 3.19, respectively. Co-injection of the cold Affibody molecule ZHER2:342 with 64Cu-DOTA-MUT-DS specifically reduced the SKOV3 tumor uptake of the probe by 48%. 111In-DOTA–MUT-DS displayed lower liver uptake at all the time points investigated and higher tumor to blood ratios at 4 and 20 h p.i., when compared with 64Cu-DOTA–MUT-DS. This study demonstrates that the 2-helix protein based probes, 64Cu/111In DOTA–MUT-DS, are promising molecular probes for imaging HER2-positive tumor. Two-helix small protein scaffold holds great promise as a novel and robust platform for imaging and therapy applications.




Similar content being viewed by others
Abbreviations
- DOTA:
-
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- PET:
-
Positron emission tomography
- SPECT:
-
Single photon emission computed tomography
- HPLC:
-
High-performance liquid chromatography
- p.i.:
-
Postinjection
- MW:
-
Molecular weight
- OSEM:
-
Ordered subsets expectation maximum
References
Ahlgren S, Wallberg H, Tran TA, Widstrom C, Hjertman M, Abrahmsen L et al (2009) Targeting of HER2-expressing tumors with a site-specifically 99mTc-labeled recombinant affibody molecule, ZHER2:2395, with C-terminally engineered cysteine. J Nucl Med 50:781–789
Andersen JT, Pehrson R, Tolmachev V, Bekele MD, Abrahmsen L, Ekblad C (2011) Extending half-life by indirect targeting of the neonatal Fc receptor (FcRn) using a minimal albumin binding domain (ABD). J Biol Chem
Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL et al (2004) Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem 47:1465–1474
Capello A, Krenning EP, Breeman WA, Bernard BF, de Jong M (2003) Peptide receptor radionuclide therapy in vitro using [111In-DTPA0]octreotide. J Nucl Med 44:98–104
Cheng Z, De Jesus O, Namavari M, De A, Levi J, Webster J et al (2008) Small-animal PET imaging of human epidermal growth factor receptor type 2 expression with site-specific 18F-labeled protein scaffold molecules. J Nucl Med 49:804–813
Ciardiello F, Tortora G (2008) EGFR antagonists in cancer treatment. N Engl J Med 358:1160–1174
Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J et al (2009) Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med 50:974–981
Ekblad T, Tran T, Orlova A, Widstrom C, Feldwisch J, Abrahmsen L et al (2008) Development and preclinical characterisation of 99mTc-labelled Affibody molecules with reduced renal uptake. Eur J Nucl Med Mol Imaging 35:2245–2255
Ellison D, Kaufman J, Mather SJ (2010) Automated radiolabelling of monoclonal antibodies with the Modular Lab system. Nucl Med Commun 31:173–177
Engel RH, Kaklamani VG (2007) HER2-positive breast cancer: current and future treatment strategies. Drugs 67:1329–1341
Ferretti G, Felici A, Papaldo P, Fabi A, Cognetti F (2007) HER2/neu role in breast cancer: from a prognostic foe to a predictive friend. Curr Opin Obstet Gynecol 19:56–62
Glisson B, Colevas AD, Haddad R, Krane J, El-Naggar A, Kies M et al (2004) HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res 10:944–946
Gotthardt M, van Eerd-Vismale J, Oyen WJ, de Jong M, Zhang H, Rolleman E et al (2007) Indication for different mechanisms of kidney uptake of radiolabeled peptides. J Nucl Med 48:596–601
Gravalos C, Jimeno A (2008) HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 19:1523–1529
Harries M, Smith I (2002) The development and clinical use of trastuzumab (Herceptin). Endocr Relat Cancer 9:75–85
Kramer-Marek G, Kiesewetter DO, Capala J (2009) Changes in HER2 expression in breast cancer xenografts after therapy can be quantified using PET and (18)F-labeled affibody molecules. J Nucl Med 50:1131–1139
Lazaridis G, Pentheroudakis G, Pavlidis N (2008) Integrating trastuzumab in the neoadjuvant treatment of primary breast cancer: accumulating evidence of efficacy, synergy and safety. Crit Rev Oncol Hematol 66:31–41
McDevitt MR, Chattopadhyay D, Jaggi JS, Finn RD, Zanzonico PB, Villa C et al (2007) PET imaging of soluble yttrium-86-labeled carbon nanotubes in mice. PLoS One 2:e907
McLarty K, Cornelissen B, Cai Z, Scollard DA, Costantini DL, Done SJ et al (2009) Micro-SPECT/CT with 111In-DTPA-pertuzumab sensitively detects trastuzumab-mediated HER2 downregulation and tumor response in athymic mice bearing MDA-MB-361 human breast cancer xenografts. J Nucl Med
McLarty K, Cornelissen B, Cai Z, Scollard DA, Costantini DL, Done SJ et al (2009b) Micro-SPECT/CT with 111In-DTPA-pertuzumab sensitively detects trastuzumab-mediated HER2 downregulation and tumor response in athymic mice bearing MDA-MB-361 human breast cancer xenografts. J Nucl Med 50:1340–1348
Miao Z, Ren G, Liu H, Jiang L, Webster JM, Zhang R et al (2011) A Novel 18F-Labeled 2-Helix Small Protein for PET Imaging of HER2 Positive Tumor. Eur J Nucl Med Mol Imaging (Submitted)
Mitra AB, Murty VV, Pratap M, Sodhani P, Chaganti RS (1994) ERBB2 (HER2/neu) oncogene is frequently amplified in squamous cell carcinoma of the uterine cervix. Cancer Res 54:637–639
Nanda R (2007) Targeting the human epidermal growth factor receptor 2 (HER2) in the treatment of breast cancer: recent advances and future directions. Rev Recent Clin Trials 2:111–116
Niu G, Li Z, Cao Q, Chen X (2009) Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with (64)Cu-DOTA-trastuzumab. Eur J Nucl Med Mol Imaging 36:1510–1519
Nygren PA (2008) Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. Febs J 275:2668–2676
Nygren PA, Skerra A (2004) Binding proteins from alternative scaffolds. J Immunol Methods 290:3–28
Orlova A, Magnusson M, Eriksson T, Nilsson M, Larsson B, Hoiden-Guthenberg I et al (2006) Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res 66:4339–4348
Orlova A, Wallberg H, Stone-Elander S, Tolmachev V (2009) On the selection of a tracer for PET imaging of HER2-expressing tumors: direct comparison of a 124I-labeled affibody molecule and trastuzumab in a murine xenograft model. J Nucl Med 50:417–425
Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC et al (2007) [64Cu-NOTA-8-Aoc-BBN(7–14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci USA 104:12462–12467
Ren G, Zhang R, Liu Z, Webster JM, Miao Z, Gambhir SS et al (2009) A 2-helix small protein labeled with 68 Ga for PET imaging of HER2 expression. J Nucl Med 50:1492–1499
Serrano-Olvera A, Duenas-Gonzalez A, Gallardo-Rincon D, Candelaria M, De la Garza-Salazar J (2006) Prognostic, predictive and therapeutic implications of HER2 in invasive epithelial ovarian cancer. Cancer Treat Rev 32:180–190
Smith-Jones P, Solit D, Akhurst T, Afroze F, Rosen N, Larson S (2004) Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol 22:701–706
Swanton C, Futreal A, Eisen T (2006) Her2-targeted therapies in non-small cell lung cancer. Clin Cancer Res 12:4377s–4383s
Tang Y, Wang J, Scollard D, Mondal H, Holloway C, Kahn H et al (2005) Imaging of HER2/neu-positive BT-474 human breast cancer xenografts in athymic mice using (111)In-trastuzumab (Herceptin) Fab fragments. Nucl Med Biol 32:51–58
Tokuda Y, Suzuki Y, Saito Y, Umemura S (2009) The role of trastuzumab in the management of HER2-positive metastatic breast cancer: an updated review. Breast Cancer
Tolmachev V (2008) Imaging of HER-2 overexpression in tumors for guiding therapy. Curr Pharm Des 14:2999–3019
Tolmachev V, Nilsson F, Widstrom C, Andersson K, Rosik D, Gedda L et al (2006) 111In-benzyl-DTPA-ZHER2:342, an affibody-based conjugate for in vivo imaging of HER2 expression in malignant tumors. J Nucl Med 47:846–853
Tolmachev V, Orlova A, Nilsson FY, Feldwisch J, Wennborg A, Abrahmsen L (2007a) Affibody molecules: potential for in vivo imaging of molecular targets for cancer therapy. Expert Opin Biol Ther 7:555–568
Tolmachev V, Orlova A, Pehrson R, Galli J, Baastrup B, Andersson K et al (2007b) Radionuclide therapy of HER2-positive microxenografts using a 177Lu-labeled HER2-specific Affibody molecule. Cancer Res 67:2773–2782
Tolmachev V, Friedman M, Sandstrom M, Eriksson T, Rosik D, Hodik M et al (2009) Affibody Molecules for Epidermal Growth Factor Receptor Targeting In Vivo: Aspects of Dimerization and Labeling Chemistry. J Nucl Med 50:274–283
Tran T, Engfeldt T, Orlova A, Sandstrom M, Feldwisch J, Abrahmsen L et al (2007) 99mTc-maEEE-ZHER2:342, an Affibody molecule-based tracer for the detection of HER2 expression in malignant tumors. Bioconjug Chem 18:1956–1964
Wallberg H, Orlova A (2008) Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: implications for development of labeled tracers. Cancer Biother Radiopharm 23:435–442
Wallberg H, Ahlgren S, Widstrom C, Orlova A (2009) Evaluation of the radiocobalt-labeled [MMA-DOTA-Cys61]-Z HER2:2395(-Cys) affibody molecule for targeting of HER2-expressing tumors. Mol Imaging Biol 12:54–62
Webster JM, Zhang R, Gambhir SS, Cheng Z, Syud FA (2009) Engineered two-helix small proteins for molecular recognition. Chembiochem 10:1293–1296
Yarden Y, Shilo BZ (2007) SnapShot: EGFR signaling pathway. Cell 131:1018
Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C (1999) From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA 96:5458–5463
Acknowledgments
This work was supported, in part, by California Breast Cancer Research Program 14IB-0091 (ZC) and SNM Pilot Research Grant (ZC), Medical Diagnostics, GE Healthcare, and National Cancer Institute (NCI) Small Animal Imaging Resource Program (SAIRP) grant R24 CA93862. We also thank Dr. Joshua Hoerner, Gregory Goddard and Hans Grade of GE Global Research for MS analysis and Dr. Alex Gibson of GE Healthcare for their helpful suggestion and reviewing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, G., Webster, J.M., Liu, Z. et al. In vivo targeting of HER2-positive tumor using 2-helix affibody molecules. Amino Acids 43, 405–413 (2012). https://doi.org/10.1007/s00726-011-1096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1096-7